
 English
English
 French
French
Isolation of autologous adipose tissue stem cells from chronic obstructive pulmonary disease patient: A single center study
Isolation des cellules souches autologues du tissu adipose des patients atteints de la bronchopulmonaire chronique obstructive: Étude mono-centre
P. Thu Phan1,2, B. Huy Nguyen1,2, T. Thanh Nguyen1,2, T. T. Thu Vu1,2, G. Van Vu1,2, K. Trong Mai2, P. Cam Pham1,2, S. Truong Ngo2, N. Viet Le2, H. Thi Chu1,2, H. Quoc Phan3, T. Huyen Tran3, Q. Ngoc Tran4, K. Ba Nguyen4, P. Ngoc Dao1,2, N. Duc Nguyen1,2, D. Thanh Dang1,2, C. Quy Ngo1,2
1: Hanoi Medical University, Vietnam.
2: Bach Mai Hospital, Hanoi, Vietnam
3: 108 Military Central Hospital, Vietnam.
4 : National Institute of Hematology and Blood Transfusion, Vietnam
DOI: 10.12699/jfvpulm.12.36.2021.23
ABSTRACT
Introduction. The stromal vascular fraction (SVF) can be isolated from the adipocyte populace and contains an abundance of adipose stem/stromal cells (ASCs). However, the safety of either ASC or SVF on chronic obstructive pulmonary disease (COPD) patients has yet been evaluated. This study aims to assess the short-term safety of the harvesting, isolating and intravenous infusion of autologous ASC from non-culture expanded SFV on COPD patients.
Methods. Participants were twenty COPD patients whose adipose tissue was harvested using standard liposuction procedure, followed by autologous ASCs of non-culture expanded SVF isolation and intravenous infusion to the patients. Primary endpoints included observing short-term adverse events during harvest, isolation and intravenous infusion ASCs. Another objective was to analyze ASCs characteristics.
Results. Of the 20 subjects, no pulmonary or cardiac serious adverse events were observed as related to the liposuction and infusion of autologous ASC from non-culture expanded SVF. Some patients reported pain and bruising at the liposuction site. Isolation of autologous adipose tissue stem cells technique ensured sterility. Harvest yielded 6.14 ± 4.08 million nucleated cells / 1 mL adipose tissue. ASCs cells expressed marker of mesenchymal stem cells (CD90, CD73, CD105). The average number of stem cells with expression of CD90, CD73, CD105 obtained from liposuction in COPD patient was 4.8 x 105 cells per 100ml of adipose tissue.
Conclusions. Autologous ASC of non-culture expanded SVF infusion was safe for COPD patients. Overall, this treatment has proved feasible in COPD treatment.
KEYWORDS: Adipose-derived stem cells; Cell therapy; COPD.
RÉSUMÉ
Introduction. La fraction vasculaire stromale (SVF) peut être isolée de la population adipocytaire et contient une abondance de cellules souches / stromales adipeuses (ASC). Cependant, l'innocuité de l'ASC ou de la SVF chez les patients atteints de bronchopulmonaire chronique obstructive (BPCO) a encore été évaluée. Cette étude vise à évaluer la sécurité à court terme de la récolte, de l'isolement et de la perfusion intraveineuse d'ASC autologue à partir de SFV étendu sans culture sur des patients atteints de BPCO.
Méthodes. Les participants étaient vingt patients atteints de BPCO dont le tissu adipeux a été prélevé en utilisant une procédure de liposuccion standard, suivie par des ASC autologues d'isolement SVF élargi sans culture et par perfusion intraveineuse aux patients. Les critères d'évaluation principaux comprenaient l'observation des événements indésirables à court terme pendant la récolte, l'isolement et les ASC en perfusion intraveineuse. Un autre objectif était d'analyser les caractéristiques des ASC.
Résultats. Sur les 20 sujets, aucun événement indésirable pulmonaire ou cardiaque grave n'a été observé comme lié à la liposuccion et à la perfusion d'ASC autologue à partir de SVF expansé sans culture. Certains patients ont signalé des douleurs et des ecchymoses au site de liposuccion. L'isolement des cellules souches du tissu adipeux autologue a assuré la stérilité. La récolte a donné 6,14 ± 4,08 millions de cellules nucléées / 1 ml de tissu adipeux. Les cellules ASC exprimaient le CD34 et le marqueur des cellules souches mésenchymateuses (CD90, CD73, CD105). Le nombre moyen de cellules souches avec expression de CD90, CD73, CD105 obtenu par liposuccion chez un patient BPCO était de 4,8 x 105 cellules pour 100 ml de tissu adipeux.
Conclusions. L'ASC autologue de la perfusion de SVF élargie sans culture était sans danger pour les patients atteints de BPCO. Dans l'ensemble, ce traitement s'est avéré faisable dans le traitement de la BPCO.
MOTS CLÉS: Cellules souches adipeuses; Thérapie cellulaire; BPCO.
INTRODUCTION
Adipose tissue stem cells (ASCs) are recognized as a sort of mesenchymal stem cell (MSC) in stromal vascular fractions (SVF) which are separated from fat tissues enzymatically. ASCs are possibly plastic-adherent in standard culture conditions. They express CD105, CD73 and CD90, but do not express CD45, CD34, CD14 [1]. The stromal vascular fraction (SVF) which is isolated from 20 - 200 mL of adipose tissue includes tens to hundreds of millions of ASC, with minimal morbidity and without requiring culture expansion. A production of ASC can be provided for infusion within 1 - 2 hour after liposuction to be suitable for practical use. More recently, stem cells’ mechanism in diseases involving significant inflammatory or degenerative factors has been detected.
Chronic obstructive pulmonary disease (COPD) is a progressive chronic disease that cannot be reversed using any known treatment. The onset and progression of smoke exposure induced emphysema have remarkably improved after intravenous infusion of cultured ASCs in a rodent model [2]. Therefore, ASCs are considered a new direction in the treatment of COPD. Nevertheless, not many studies to date have evaluated the safety and effectiveness of ASCs in COPD patients. The main objective of this study is to evaluate the short-term safety of harvesting, isolating and intravenously infusing autologous ASC from non-culture expanded SFV on COPD patients.
METHODS
The clinical trial design was reviewed and approved for conduct at Bach Mai Hospital in Hanoi, Vietnam, by the Ministry of Science and Technology and the Research Ethics Committee of the Ministry of Health of Viet Nam. All subjects gave written informed consent.
Inclusion and exclusion criteria
Participants in the study adhered to strict inclusion and exclusion criteria.
Inclusion criteria including: 1) age 40 - 80 years old; 2) a diagnosis of COPD according to the 2016 Global Initiative for Chronic Obstructive Pulmonary Disease (GOLD) (3); and 3) FEV1 ≤ 60% and ≥ 2 exacerbations or ≥ 1 leading to hospital admission in previous year.
Exclusion criteria were follows: 1) other lung diseases; 2) α1- antitrypsin deficiency; 3) weight < 40kg; 4) presence of active infectious diseases or acute exacerbation or hospitalization within the previous 4 weeks; 5) presence of active malignancy; 6) smoking or just quitting smoking for 6 months; 7) being pregnant; 8) evidence of other organ pathology as determined by abnormal laboratory values; 9) using immunosuppressants for at least 8 weeks; 10) using TNF inhibitors within the previous 3 months; 11) history of drug allergy; 12) having any pathology that assessed survival < 6 months; 13) inability to carry out the necessary clinical and evaluation tests; and 14) participating in another study. Participants were selected from August 2018 to December 2020. Their medical history was assessed. Furthermore, a physical examination was performed. A lung CT scan, pulmonary function test and full laboratory studies were also obtained to assess pre-procedural heart, liver, kidney, and metabolic parameters.
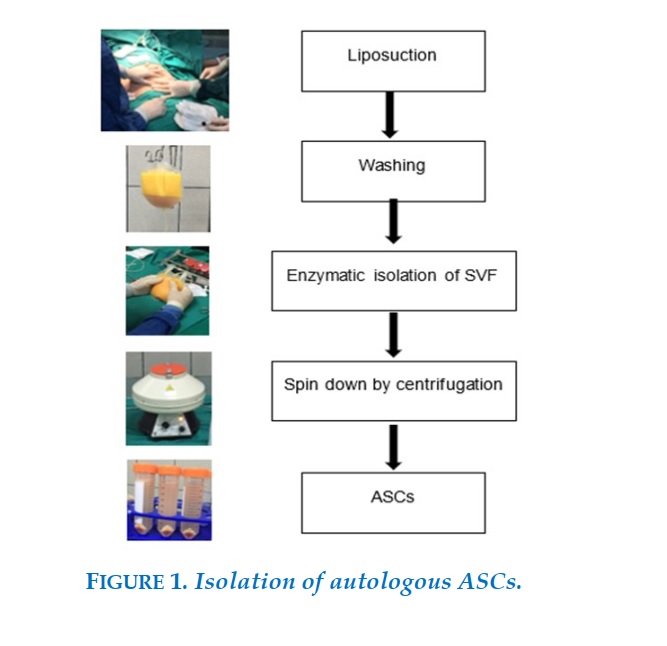
Isolation of autologous adipose tissue stem cells
Liposuction was done by a plastic surgeon in the operating room. Under sterile conditions, 600 - 700 ml tumescent solution (1-L saline with 20 mL of 1% lidocaine and 1 mg of epinephrine) was infiltrated into the abdomen. After appropriate time for anesthetic, fat was removed. The fat was then transferred to the sterile processing facility. Tumescent solution was removed from the fat in an approximately one-hour process. It then took 50 minutes to digest adipose tissue with enzyme (Adistem Large Kit). After repetitive wash and centrifugation steps (3 - 4 times), the resulting SVF was resuspended into 12 ml of 0.9% NaCl saline (Figure 1).
Platelet-rich plasma solution was produced using Adistem's PRP kit to process 20 ml of whole blood. The SVF was divided into two parts.
One part was mixed with a rich platelet solution and light-activated using AdiStem™ AdiLight LED then mixed with 100 ml of 0.9% NaCl saline and infused for 30 minutes intravenously via the antecubital vein. The other part was mixed with PRP and DMSO and cryopreserved at -196oC during 6 months for a second intravenous infusion into the patient. The patients were followed up in the next 12 months.
Measured short-term safety
During time of harvest, isolation and intravenous infusion of autologous ASC from non-culture expanded SFV, the participants were continuously monitored via pulse oximetry and electrocardiography for any evidence of cardiac or pulmonary instability. Post-liposuction, they were followed up on pain (VAS), bruising, bleeding, infection, ... and accidents during stem cell infusion.
Autologous ASC of non-culture expanded SFV was assessed on cellular viability using Trypan blue. The number of nucleus cells was estimated using automated hematology analyzers. Surface marking of autologous ASC was detected using flow cytometry. All samples were cultured for infection.
RESULTS
Baseline patients’ characteristics
All participants were over 50 years old and male. Almost all subjects had severe and very severe level of airway obstruction. Subjects had GOLD C and GOLD D COPD based on GOLD classification in 2016. Smoking was the main environmental contributor to COPD in this population (Table 1).
The short-term safety of ASC harvesting, isolating and intravenous infusion
Table 2 presents that no serious abnormal incidents were observed. The vital parameters (pulse, blood pressure, temperature, breathing rate, SpO2) were stable during liposuction.
Almost all patients felt a mild pain in the liposuction area (VAS <3 points) for about 2 days after the procedure.
Some patients have bruises from the liposuction which went away 7-10 days after the procedure. No adverse events, including anaphylaxis, electrocardiogram abnormalities, rhythm disturbances (including bradycardia or tachycardia), oxygen desaturation, fever (temperature > 38.6 °C), or subjective feelings of discomfort, were noted in relation to the infusion.
Autologous ASC of non-culture expanded SFV characteristics
Table 3 shows that fat tissue obtained ranges between 70ml and 180 ml during the liposuction procedure. Nucleated cells were for a yield of 6.14 ± 4.08 million nucleated cells/mL. Flow cytometric analysis showed that ASCs cells isolated using our method expressed CD90, CD73, CD105 of mesenchymal stem cells. The results of cultures for infection were negative.
DISCUSSION
While several studies have reported the use of stem cells from adipose tissue on patients with respiratory disease such as idiopathic pulmonary fibrosis [4] and acute respiratory distress syndrome [5], data on COPD treatment by ASCs has been lacking [6, 7]. COPD is a consistently progressive, eventually fatal disease for which no treatment has proved competent of either reversing or even interrupting its course. However, the known primary mechanisms of MSC in COPD include inhibition of excessive inflammatory response, correction of Protease-resistant imbalance, inhibition of alveolar apoptosis, changes in oxidative stress, epithelial repair of lung tissue and endothelium, antibacterial and reduction in pulmonary arterial pressure [2, 8-15]. This suggests a potential direction in the treatment of COPD by stem cell.
What distinguishes this study from previous ones is the focus on elderly patients with chronic lung disease.
Almost all patients with severe and very severe COPD had average weight and the subcutaneous fat was significant. The liposuction site was, thus, mainly in the abdomen. The U.S has taken approximately 400,000 liposuction surgeries each year and these procedures yield anywhere from 100 ml to >3 L of lipoaspirate tissue [16]. However, this number was from 50 – 200ml in COPD patients. The isolation processes of ASCs have been created and optimized for 20 years to be more consistent with therapeutic application. This enzymatic isolation procedure is most valuable and effective for clinical use. Raposio et al. (2017) developed the standard protocol for isolation of ASCs that showed the maximum number of stem cells obtained from liposuction (9.06 x 105 cells per 100mL of adipose tissue) with 99% cell vitality [17]. In our study, the average number of stem cells with expression of CD90, CD73, CD105 obtained from liposuction in COPD patient was 4.8 x 105 cells per 100ml of adipose tissue. ASCs are isolated from SVF. Flow cytometry is helpful for the classification of ASCs based on specific markers. Expression of those markers is dependent on isolation method, time incubation, and culture condition [18].
The safety of ASCs in the treatment of various diseases has been confirmed for years [19]. However, carefully preparation before the intervention was required because the patients in this study had severe and very severe COPD and thus, were at a higher risk of respiratory failure or cardiovascular events during liposuction and infusion of ASCs. The patient had no exacerbation in the previous month and was in stable respiratory condition. Good local anesthesia contributed to the patient's resting position, relieved pain and reduced the complications of liposuction. Patients were monitored during liposuction and infusion of autologous stem cells from adipose tissue and followed up for 7 days in hospital. Main complications were pain and bruising at the liposuction site which have not affected the dyspnea of COPD patients. No observed pulmonary or cardiac issues related to the procedure were observed.
This report is a foundation for future evaluations of ASCs safety and effectiveness in COPD treatment by providing the evidence on the feasibility and safety of intravenous infusion of autologous ASCs of non-culture expanded SVF immediately following lipoaspiration.
CONCLUSION
This study has evaluated the safety of autologous ASCs harvesting, isolation and infusion on severe to very severe COPD patients. Isolation of autologous adipose tissue stem cells from COPD patients was performed without any adverse events. Autologous ASC of non-culture expanded SVF infusion was found to be safe for COPD patients. Although the sample size was small with only 20 patients, this study contributed to the knowledge of COPD treatment with autologous stem cell from adipose tissue.
Data sharing statement
Participant data (sociodemographic variables [age, sex, and GOLD grade) used in the analysis in this study, together with a complementary specification of the intervention can be obtained in direct request to the corresponding author.
Acknowledgments
This project was supported by the Ministry of Science and Technology, 108 Military Central Hospital and National Institute of Hematology and Blood Transfusion, Hanoi, Viet Nam.
CONFLICT OF INTERESTS
Non.
REFERENCES
1. Dominici M LBK, Mueller I, et al,. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315-7.
2. Schweitzer KS JB, Garrison J, Rush NI, Cooper S, Traktuev DO, Feng D, et al,. Adipose stem cell treatment in mice attenuates lung and systemic injury induced by cigarette smoking. Am J Respir Crit Care Med. 2011;183(2):215-25.
3. GOLD. Global strategy for diagnosis, management and prevention of COPD. NHLBI/WHO workshop report. 2016.
4. Tzouvelekis VP, George Koliakos, Paschalis Ntolios,. A prospective, non-randomized, no placebo-controlled, phase Ib clinical trial to study the safety of the adipose derived stromal cells-stromal vascular fraction in idiopathic pulmonary fibrosis. Journal of Translational Medicine. 2013;11:171
5. Guoping Zheng LH, Haijiang Tong, Qiang Shu, Yaoqin Hu, Menghua Ge, Liuya Zhang, Bin Zou, Baoli Cheng, Jianguo Xu. Treatment of acute respiratory distress syndrome with allogeneic adipose-derived mesenchymal stem cells: a randomized, placebo-controlled pilot study. Respiratory Research. 2014;15:39.
6. Weiss DJ CR, Flannery R, LeRoux-Williams M, Tashkin DP,. A placebo-controlled, randomized trial of mesenchymal stem cells in COPD. Chest. 2013;143(6):1590-8.
7. Kristin Comella JAPB, Tom Ichim, Javier Lopez, Jose Limon, Ruben Corral Moreno,. Autologous stromal vascular fraction in the intravenous treatment of end-stage chronic obstructive pulmonary disease: A phase I trial of safety and tolerability. J Clin Med Res. 2017;9(8):701-8.
8. Neufeld G, Tzafra Cohen, Stela Gengrinovitch et al. Vascular endothelial growth factor (VEGF) and its receptors. The FASEB Journal. 1999;13(1): 9-22.
9. Qin ZH et al. Intrapleural delivery of MSCs attenuates acute lung injury by paracrine/endocrine mechanism. J Cell Mol Med. 2012;16(11):2745-53.
10. Sotiropoulou PA SP, AD Gritzapis et al. Interactions between human mesenchymal stem cells and natural killer cells. Stem Cells. 2006;24(1):74-85.
11. Alon T, Itzhak Hemo, Ahuva Itin et al. Vascular endothelial growth factor acts as a survival factor for newly formed retinal vessels and has implications for retinopathy of prematurity. Nature medicine. 1995;1(10):1024-8.
12. Gerber H-P, Amy McMurtrey, Joe Kowalski et al. Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 39-kinase/Akt signal transduction pathway: requirement for Flk-1/KDR activation. Journal of Biological Chemistry. 1998;273:30336–43.
13. Furuya N, Mitsuko Takenaga, Yuki Ohta et al. Cell therapy with adipose tissue-derived stem/stromal cells for elastase-induced pulmonary emphysema in rats. Regenerative medicine. 2012;7(4):503-12.
14. Shigemura N, Yoshiki Sawa, Shinya Mizuno et al. Amelioration of pulmonary emphysema by in vivo gene transfection with hepatocyte growth factor in rats. Circulation. 2005;111(11):1407-14.
15. Shigemura N OM, Mizuno S et al,. Autologous transplantation of adipose tissue‐derived stromal cells ameliorates pulmonary emphysema. American journal of transplantation. 2006;6(11):2592-600.
16. A.J. Katz RL, M.H. Hedrick, J.W. Futrell. Clin Plast Surg. 1999;26:587-603.
17. Raposio EB, N. Isolation of Ready-to-Use Adipose-Derived Stem Cell (ASC) Pellet for Clinical Applications and a Comparative Overview of Alternate Methods for ASC Isolation. Curr Protoc Stem Cell Biol. 2017;41:1F-17.
FIGURE - TABLES
REFERENCES
1. Dominici M LBK, Mueller I, et al,. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315-7.
2. Schweitzer KS JB, Garrison J, Rush NI, Cooper S, Traktuev DO, Feng D, et al,. Adipose stem cell treatment in mice attenuates lung and systemic injury induced by cigarette smoking. Am J Respir Crit Care Med. 2011;183(2):215-25.
3. GOLD. Global strategy for diagnosis, management and prevention of COPD. NHLBI/WHO workshop report. 2016.
4. Tzouvelekis VP, George Koliakos, Paschalis Ntolios,. A prospective, non-randomized, no placebo-controlled, phase Ib clinical trial to study the safety of the adipose derived stromal cells-stromal vascular fraction in idiopathic pulmonary fibrosis. Journal of Translational Medicine. 2013;11:171
5. Guoping Zheng LH, Haijiang Tong, Qiang Shu, Yaoqin Hu, Menghua Ge, Liuya Zhang, Bin Zou, Baoli Cheng, Jianguo Xu. Treatment of acute respiratory distress syndrome with allogeneic adipose-derived mesenchymal stem cells: a randomized, placebo-controlled pilot study. Respiratory Research. 2014;15:39.
6. Weiss DJ CR, Flannery R, LeRoux-Williams M, Tashkin DP,. A placebo-controlled, randomized trial of mesenchymal stem cells in COPD. Chest. 2013;143(6):1590-8.
7. Kristin Comella JAPB, Tom Ichim, Javier Lopez, Jose Limon, Ruben Corral Moreno,. Autologous stromal vascular fraction in the intravenous treatment of end-stage chronic obstructive pulmonary disease: A phase I trial of safety and tolerability. J Clin Med Res. 2017;9(8):701-8.
8. Neufeld G, Tzafra Cohen, Stela Gengrinovitch et al. Vascular endothelial growth factor (VEGF) and its receptors. The FASEB Journal. 1999;13(1): 9-22.
9. Qin ZH et al. Intrapleural delivery of MSCs attenuates acute lung injury by paracrine/endocrine mechanism. J Cell Mol Med. 2012;16(11):2745-53.
10. Sotiropoulou PA SP, AD Gritzapis et al. Interactions between human mesenchymal stem cells and natural killer cells. Stem Cells. 2006;24(1):74-85.
11. Alon T, Itzhak Hemo, Ahuva Itin et al. Vascular endothelial growth factor acts as a survival factor for newly formed retinal vessels and has implications for retinopathy of prematurity. Nature medicine. 1995;1(10):1024-8.
12. Gerber H-P, Amy McMurtrey, Joe Kowalski et al. Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 39-kinase/Akt signal transduction pathway: requirement for Flk-1/KDR activation. Journal of Biological Chemistry. 1998;273:30336–43.
13. Furuya N, Mitsuko Takenaga, Yuki Ohta et al. Cell therapy with adipose tissue-derived stem/stromal cells for elastase-induced pulmonary emphysema in rats. Regenerative medicine. 2012;7(4):503-12.
14. Shigemura N, Yoshiki Sawa, Shinya Mizuno et al. Amelioration of pulmonary emphysema by in vivo gene transfection with hepatocyte growth factor in rats. Circulation. 2005;111(11):1407-14.
15. Shigemura N OM, Mizuno S et al,. Autologous transplantation of adipose tissue‐derived stromal cells ameliorates pulmonary emphysema. American journal of transplantation. 2006;6(11):2592-600.
16. A.J. Katz RL, M.H. Hedrick, J.W. Futrell. Clin Plast Surg. 1999;26:587-603.
17. Raposio EB, N. Isolation of Ready-to-Use Adipose-Derived Stem Cell (ASC) Pellet for Clinical Applications and a Comparative Overview of Alternate Methods for ASC Isolation. Curr Protoc Stem Cell Biol. 2017;41:1F-17.
ARTICLE INFO
DOI: 10.12699/jfvpulm.12.36.2021.23
Conflict of Interest
Non
Date of manuscript receiving
15/12/2020
Date of publication after correction
15/01/2021
Article citation
Thu Phan P., Huy Nguyen B., Thanh Nguyen T., Thu Vu T. T., Van Vu G., Trong Mai K., Cam Pham P., Truong Ngo S., Viet Le N., Thi Chu H., Quoc Phan H., Huyen Tran T., Ngoc Tran Q., Ba Nguyen K., Ngoc Dao P., Duc Nguyen N., Thanh Dang D., Quy Ngo C. Isolation of autologous adipose tissue stem cells from chronic obstructive pulmonary disease patient: A single center study. J Func Vent Pulm 2021;36(12):23-28