
 English
English
 French
French
Warthin’s tumor associated with lung adenocarcinoma: A case report
Tumeur de Warthin associée à un adénocarcinome pulmonaire: Un rapport de cas
S. Naciri, R. Zahraoui, M. Soualhi, J-E. Bourkadi
Moulay Youssef Hospital, Pulmonary Unit, University Mohammed V of Rabat, Morocco
Corresponding author:
Dr. Salim Naciri
Service de Pneumologie, Hôpital Moulay Youssef, CHU Ibn Sina, Faculté de Médecine et de Pharmacie, Université Mohammed V, Rabat, Maroc.
E-mail: naciri_salim@hotmail.com
DOI: 10.12699/jfvpulm.12.36.2021.60
Introduction. Warthin’s tumour, also known as adenolymphoma or papillary lymphomatous cystadenoma, is the second most common benign neoplasm of the parotid gland. The coexistence of lung cancer and WT is reported very rarely in the literature.
Case presentation. We report the case of a 50-year-old male smoker, to the pulmonology department with complaints of right chest pain throughout the previous two months. He also had noticed a swelling on the left side of the neck 5 years previously, but the lesion rapidly enlarged with associated pain 2 months previously. Histopathological examination showed typical focal features of Whartin’s tumour. A radiological examination that revealed a lesion with spiculation in the upper lobe of the right lung. A transthoracic biopsy was performed and revealed a lung adenocarcinoma.
Conclusion. This report discusses the diagnosis of a rare case of synchronous lesions of WT and lung cancer in the light of similar cases sporadically reported in literature.
KEYWORDS: Warthin’s tumour; Lung adenocarcinoma.
Introduction. La tumeur de Warthin, également appelée adénolymphome ou cystadénome lymphomateux papillaire, est le deuxième néoplasme bénin le plus fréquent de la glande parotide. La coexistence du cancer du poumon et du WT est très rarement rapportée dans la littérature.
Présentation du cas. Nous rapportons le cas d'un fumeur de 50 ans au service de pulmonologie avec des plaintes de douleur thoracique droite au cours des deux mois précédents. Il avait également remarqué un gonflement du côté gauche du cou 5 ans auparavant, mais la lésion s'est rapidement agrandie avec une douleur associée 2 mois auparavant. L’examen histopathologique a montré les caractéristiques focales typiques de la tumeur de Whartin. Un examen radiologique qui a révélé une lésion avec spiculation dans le lobe supérieur du poumon droit. Une biopsie transthoracique a été réalisée et a révélé un adénocarcinome pulmonaire.
Conclusion. Ce rapport discute du diagnostic d'un cas rare de lésions synchrones de WT et de cancer du poumon à la lumière de cas similaires rapportés sporadiquement dans la littérature.
MOTS CLÉS: Tumeur de Warthin; Adénocarcinome pulmonaire.
INTRODUCTION
Warthin’s tumour, also known as adenolymphoma or papillary lymphomatous cystadenoma, is the second most common benign neoplasm of the parotid gland. The coexistence of lung cancer and WT is reported very rarely in the literature.
CASE PRESENTATION
A 50-year-old man presented to the pulmunology department with complaints of right chest pain throughout the previous two months. He had a history of 20 pack/year of cigarette consumption associated with chronic cough.
He also had noticed a swelling on the left side of the neck 5 years previously, but the lesion rapidly enlarged with associated pain 2 months previously.
His physical examination showed subcutaneously mass of 2 x 5cm size below the left mandibular angle. Also, two small lymphadenopathies were present on the right cervical side. We noticed a bilateral digital hippocratism.
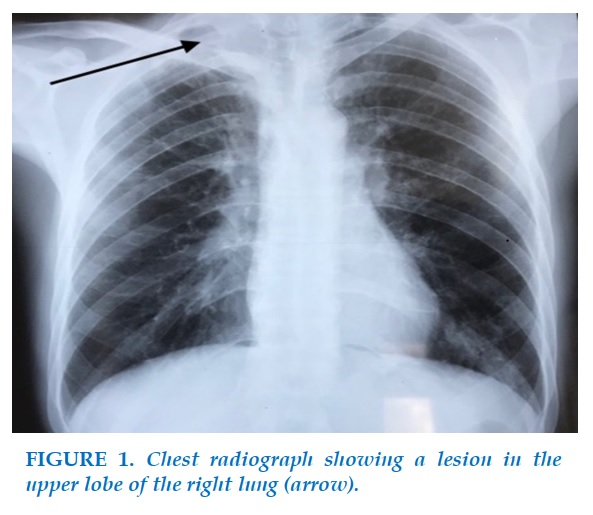
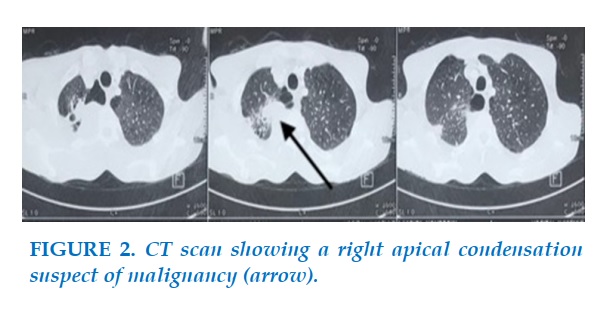
A chest radiograph (Figure 1) revealed a lesion in the upper lobe of the right lung, malignant looking at the chest CT scan, even without visible mediastinal lymphadenopathy (Figure 2).
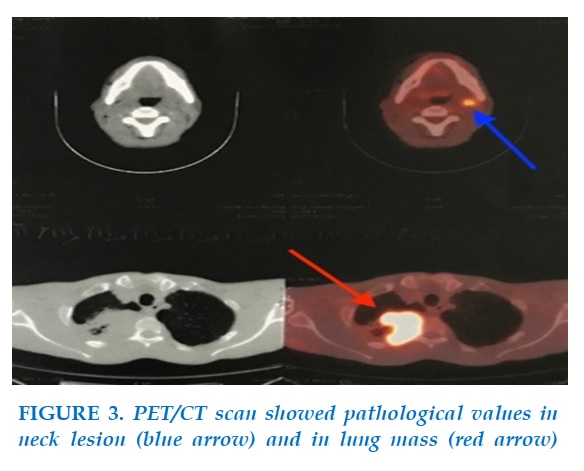
Considering that the lesion in the neck could be a metastatic lymph gland, we performed a PET/CT scan that carried out yielded pathological 18FDG values of 22,8 and 8,4, respectively, for the mass seen in the lung and the neck (Figure 3). The PET/CT scan also revealed cervical and right hilar pulmonary lymph nodes, as well as bilateral lung nodules.
It was decided to perform an ultrasound-guided cytological examination of the parotid that revealed a WT.
Following the nondiagnostic fiberoptic bronchoscopy, transthoracic percutaneous fine needle aspiration biopsy documented a lung adenocarcinoma (immunohistochemically: TTF1+, CK7+, P63-, CK5/6-, CK20-) (T4N2M1a, stage IV), and palliative care with no surgical intervention indicated.
DISCUSSION
Salivary gland neoplasms represents a relatively rare entity, estimated at 3-5% of all the head and neck neoplasms [1,2].
WT, also known as adenolymphoma or papillary lymphomatous cystadenoma, is the second most common benign neoplasm of the parotid gland, comprising 15% of the parotid epithelial tumors [3].
It has been well documented that smokers have an increased risk of developing WT [4-8]. As smokers have also an increased risk of lung cancer, the close association of smoking with both tumours can likely explains concomitant diagnosis. In fact, White and al’s retrospective chart review [9] reported 24 of 144 (19%) patients with WT who had synchronous lung cancer, the most common being non-small cell lung cancer and stage I or IV, as observed in our case. Another recent study by Zaccarini and al found a 10% association rate (7 cases out of 73) [8]. Zaccarini suggested that the prevalence of secondary non-salivary neoplasms in patients harboring WT is underestimated given that he found a 37% association rate for all cancers (including pulmonary). Seifert and all's retrospective study found a rate of 3% (8 cases out of 275) [10]. This association is understandable considering smoking risk factor, but there is debate regarding the development of WT.
The first concept supports that pulmonary localization is nothing other than a metastatic localization of WT. Histologically, WT typically has oncocytic glandular structures and a lymphoid stroma [11]. The potential for malignant transformation of the WT is exceptional (0,1%-1%) and concerns both epithelial or lymphoid component [12]. Seifert and al [10] mentioned in their study a rare case of malignant transformation at the stage of hematogenous dissemination with carcinomatous locations of the lung, skin and liver. This case of lung metastasis from a WT was reported by Kessler and al in 1977 [13] and is based on autopsy findings. However, this theory does not seem suitable for our case in the light of immunohistochemistry in favor of a primary pulmonary lesion (TTF1 and CK7 positive).
The second theory, which may seem more suited to our case, can be interpreted in reverse, that is, WT could be a form of expression of a metastasis of primary lung cancer, since the ethiopathogenesis of WT remains unclear. There has been one reported case of lung adenocarcinoma that metastasized to WT [14] based on the histological appearance and immunohistochemical profile, given the expression of TTF1 in both tissues and knowing that primary tumors of salivary gland are always TTF1 negative. In view of the findings of this interesting case, we regret the non-realization of an immunohistochemical study on the fine needle aspiration of the parotid, especially since our case was similar with a positive TTF1 marker (primary lung adenocarcinoma). Other molecular research along the same lines suggested that salivary gland tumors often have strong EGFR protein expression, and some patients may benefit from EGFR directed therapy [15].
This research provides further evidence of the close relationship between WT and lung malignancies, and new studies should be conducted to assess WT on a molecular level.
However, this theory also seems implausible to us considering the fact that, in our case, the parotid lesion had been present for years prior to the diagnosis, and as the mass was painless, and not bothersome, it was not addressed until chest pain commenced. In fact, it is in our opinion rare to find a metastasis of lung cancer evolving over 5 years with a general condition relatively well preserved. However, although WT itself is a benign lesion, it is essential to recognize and be vigilant of the high risk of lung cancer in those patients, and to offer continued, close surveillance.
At last, our study has another limitation, regarding fine needle aspiration. Indeed, according to the most recent series, the sensitivity varies from 73% to 93% and the specificity from 85% to 98% [5,16,17]. The false negatives of fine needle aspiration are, among other things, inherent in the very heterogeneous polymorphic nature of the vast majority of carcinomas of the salivary glands. False positives have been reported in cytology between the diagnosis of cystadenolymphoma (WT) and that of intra-parotid lymph node metastasis of an epidermoid carcinoma [18].
CONCLUSION
To date, there has been no proven relationship between WT and lung cancer. Even if the coexistence of lung cancer and WT is reported very rarely in the literature, more studies should be carried out to prove this close relationship on the ethiopathogenic and molecular level. Finally, it is useful to judge that patients with WT are at high risk of developing lung cancer, and therefore earlier recognition of these benign lesions may potentially facilitate earlier diagnosis of the lung malignancy, leading to reductions in patient morbidity and mortality.
CONFLIT OF INTEREST
Non.
REFERENCES
1. Califano J, Eisele DW. Benign salivary gland neoplasms. Otolaryngol Clin North Am1999;32(5):861–73.
2. Bonfils P. Tumori delle ghiandole salivari. EMC - Otorinolaringoiatr2007;6(4):1–17.
3. Dubrulle F, Martin-Duverneuil N et Moulin G, Imagerie en ORL, Elsevier-Masson2010.
4. Chedid HM, Rapoport A, Aikawa Kf, Menezes Ad, and al. Warthin's tumor of the parotid gland: study of 70 cases. Rev Col Bras Cir 2011;38(2):90-4.
5. Paris J, Facon F, Chrestian MA, Giovanni A, Zanaret M. Approche diagnostique des tumeurs de Warthin : clinique, cytoloponction et IRM. Rev LaryngolOtolRhinol (Bord) 2004;125:65-9.
6. Teymoortash A, Krasnewicz Y, Werner JA. Clinical features of cystadenolymphoma (Warthin’s tumor) of the parotid gland: A retrospective comparative study of 96 cases. Oral Oncol 2006;42:569-73.
7. Yu Gy, Liu XB, Li ZL, Peng X. Smoking and the development of Warthin’s tumour of the parotid gland. Br J Oral Maxillofac Surg 1998; 36:183-5.
8. Zaccarini DJ, Khurana KK. Incidence of Non-Salivary Gland Neoplasms in Patients with Warthin Tumor: A Study of 73 Cases. Head Neck Pathol 2019 Jun 21.
9. White CK, Williams KA, Rodriguez-Figueroa J, Langer CJ. Warthin's tumors and their relationship to lung cancer. Cancer Invest 2015 Jan;33(1):1-5.
10. Seifert G, Bull HG, Donath K. Histologic subclassification of the cystadenolymphoma of the parotid gland. Analysis of 275 cases. Virchows Arch A Pathol Anat Histol 1980;388:13-38.
11. Just PA, Miranda L, Elouaret Y, Meatchi T, Hans S, Badoual C. Classification des tumeurs des glandes salivaires. Ann Otolaryngol Chir Cervicofac 2008; 125:331-40.
12. Nagao T, Sugano I, Ishida Y, Tajima Y, and al. Mucoepidermoid carcinoma arising in Warthin’stumour of the parotid gland: report of two cases with histopathological, ultrastructural and immunohistochemical studies. Histopathology 1998; 33:379-86.
13. Kessler E, Koznizky I L, and Schindel J. Malignant Warthin’s tumor. Oral Surgery. Oral Medicine, Oral Pathology 1977;43(1), 111–115.
14. Laco J, Celakovsky P, Kalfert D, Hornychova H, Rybnikar T, Ryska A. Tumor-to-tumor metastasis: Warthin tumor as a recipient of lung carcinoma and of renal carcinoma - Report of two cases. Pathol Res Pract 2010 Jul 15;206(7):458-62.
14. Clauditz TS, Gontarewicz A, Lebok P, Tsourlakis MC, Grob TJ, Münscher A, Sauter G, Bokemeyer C, Knecht R, Wilczak W. Epidermal growth factor receptor (EGFR) in salivary gland carcinomas: potentials as therapeutic target. Oral Oncol 2012 Oct;48(10):991-996.
16. Jafari A, Royer B, Lefevre M, Corlieu P, and al. Value of the cytological diagnosis in the treatment of parotid tumors. Otolaryngol Head Neck Surg 2009;140:381-5.
17. Baglin AC, Wassef M. Cytoponction des glandes salivaires : le pour et le contre. Ann Pathol 2007;27:1S78-80.
18. Jan IS, Chung PF, Weng MH, Huang MS, Lee YT, Cheng TY, Ro JY, Kuo SH. Analysis of fine-needle aspiration cytology of the salivary gland. J Formos Med Assoc 2008;107(5):364-70.
-FIGURES-
REFERENCES
1. Califano J, Eisele DW. Benign salivary gland neoplasms. Otolaryngol Clin North Am1999;32(5):861–73.
2. Bonfils P. Tumori delle ghiandole salivari. EMC - Otorinolaringoiatr2007;6(4):1–17.
3. Dubrulle F, Martin-Duverneuil N et Moulin G, Imagerie en ORL, Elsevier-Masson2010.
4. Chedid HM, Rapoport A, Aikawa Kf, Menezes Ad, and al. Warthin's tumor of the parotid gland: study of 70 cases. Rev Col Bras Cir 2011;38(2):90-4.
5. Paris J, Facon F, Chrestian MA, Giovanni A, Zanaret M. Approche diagnostique des tumeurs de Warthin : clinique, cytoloponction et IRM. Rev LaryngolOtolRhinol (Bord) 2004;125:65-9.
6. Teymoortash A, Krasnewicz Y, Werner JA. Clinical features of cystadenolymphoma (Warthin’s tumor) of the parotid gland: A retrospective comparative study of 96 cases. Oral Oncol 2006;42:569-73.
7. Yu Gy, Liu XB, Li ZL, Peng X. Smoking and the development of Warthin’s tumour of the parotid gland. Br J Oral Maxillofac Surg 1998; 36:183-5.
8. Zaccarini DJ, Khurana KK. Incidence of Non-Salivary Gland Neoplasms in Patients with Warthin Tumor: A Study of 73 Cases. Head Neck Pathol 2019 Jun 21.
9. White CK, Williams KA, Rodriguez-Figueroa J, Langer CJ. Warthin's tumors and their relationship to lung cancer. Cancer Invest 2015 Jan;33(1):1-5.
10. Seifert G, Bull HG, Donath K. Histologic subclassification of the cystadenolymphoma of the parotid gland. Analysis of 275 cases. Virchows Arch A Pathol Anat Histol 1980;388:13-38.
11. Just PA, Miranda L, Elouaret Y, Meatchi T, Hans S, Badoual C. Classification des tumeurs des glandes salivaires. Ann Otolaryngol Chir Cervicofac 2008; 125:331-40.
12. Nagao T, Sugano I, Ishida Y, Tajima Y, and al. Mucoepidermoid carcinoma arising in Warthin’stumour of the parotid gland: report of two cases with histopathological, ultrastructural and immunohistochemical studies. Histopathology 1998; 33:379-86.
13. Kessler E, Koznizky I L, and Schindel J. Malignant Warthin’s tumor. Oral Surgery. Oral Medicine, Oral Pathology 1977;43(1), 111–115.
14. Laco J, Celakovsky P, Kalfert D, Hornychova H, Rybnikar T, Ryska A. Tumor-to-tumor metastasis: Warthin tumor as a recipient of lung carcinoma and of renal carcinoma - Report of two cases. Pathol Res Pract 2010 Jul 15;206(7):458-62.
14. Clauditz TS, Gontarewicz A, Lebok P, Tsourlakis MC, Grob TJ, Münscher A, Sauter G, Bokemeyer C, Knecht R, Wilczak W. Epidermal growth factor receptor (EGFR) in salivary gland carcinomas: potentials as therapeutic target. Oral Oncol 2012 Oct;48(10):991-996.
16. Jafari A, Royer B, Lefevre M, Corlieu P, and al. Value of the cytological diagnosis in the treatment of parotid tumors. Otolaryngol Head Neck Surg 2009;140:381-5.
17. Baglin AC, Wassef M. Cytoponction des glandes salivaires : le pour et le contre. Ann Pathol 2007;27:1S78-80.
18. Jan IS, Chung PF, Weng MH, Huang MS, Lee YT, Cheng TY, Ro JY, Kuo SH. Analysis of fine-needle aspiration cytology of the salivary gland. J Formos Med Assoc 2008;107(5):364-70.