
 English
English
 French
French
Study of asthma control status in children with bronchial asthma and allergic rhinitis
Recherche sur le statut de contrôle de l'asthme chez les enfants atteints d'asthme et de rhinite allergique
H. Nguyen-Tran-Ngoc1, Th. Nguyen-Thi-Dieu2, D. Tran-Van1, D. Luong-Cao1, S. Duong-Quy3
1 : Vietnam Military Medical University. Hanoi, Vietnam
2: Hanoi Medical University. Hanoi, Vietnam
3: Lam Dong Medical College. Dalat, Vietnam
Corresponding author
Pr. DUONG-QUY Sy
Lam Dong Medical College
E-mail: sduongquy.jfvp@gmail.com
DOI: 10.12699/jfvpulm.12.37.2021.13
Introduction. Asthma and allergic rhinitis are the most common chronic airway diseases in children. According to the Association of Allergic Rhinitis and its Impact on Asthma - ARIA (Allergic Rhinitis and its Impact on Asthma), the rate of allergic rhinitis (AR) with bronchial asthma accounts for 80%. Studies have confirmed the important role of bronchial asthma control in AR control outcomes in asthmatic patients with AR. Studies have shown that bronchial asthma worsens ARand that AR treatment improves asthma symptoms. We conducted this study with the expectation of evaluating the correlations between NO levels and asthma control status.
Methods. A cross-sectional descriptive study on 154 subjects volunteered to participate in the study and was divided into 2 groups: group 1 consisted of 124 children with asthma and a co-morbid condition called AR; Group 2 consisted of 30 subjects as a control group with children with bronchial asthma but had no associated AR.
Results. The study results showed that the concentration of nNO in children with asthma with allergic rhinitis was higher than that in children with asthma without allergic rhinitis. The concentration of nNO is closely related to FENO and allergic factors such as IgE, eosinophils in peripheral blood. After 6 months of prophylactic treatment, the rate of complete asthma control in children with asthma was lowest according to GINA criteria of 67.5%, and highest according to ACT criteria at 96.1%. Airway FeNO and nNO concentrations decreased after asthma and allergic rhinitis prophylaxis. Parameters of blood eosinophils, blood IgE, FeNO, and nNO are inflammatory markers that help classify asthma phenotype, predict severity of asthma and predict/ expect a response to treatment with corticosteroids.
KEYWORDS: Asthma; Allergic rhinitis; Nitric oxide; NO; FeNO; Nasal NO.
Introduction. L'asthme et la rhinite allergique sont les maladies chroniques des voies respiratoires les plus courantes chez les enfants. Selon l'Association of Allergic Rhinitis and its Impact on Asthma-ARIA (Allergic Rhinitis and its Impact on Asthma), le taux d'rhinite allergique (RA) avec asthme bronchique représente 80%. Des études ont confirmé le rôle important du contrôle de RA dans le contrôle de l'asthme. résultats chez les patients asthmatiques souffrant de RA. Des études ont montré que l'asthme bronchique aggrave la RA et que le traitement RA améliore les symptômes de l'asthme. Nous avons mené cette étude afin d’évaluer les corrélations entre les niveaux de NO et l'état de contrôle de l'asthme.
Méthodes. Une étude descriptive transversale sur 154 sujets se sont portés volontaires pour participer à l'étude et a été divisée en 2 groupes: le groupe 1 se composait de 124 enfants souffrant d'asthme et d'une comorbidité appelée rhinite allergique; Le groupe 2 était composé de 30 sujets en tant que groupe témoin avec des enfants souffrant d'asthme bronchique mais n'avait pas de rhinite allergique associée.
Résultats. Les résultats de l'étude ont montré que la concentration de nNO chez les enfants asthmatiques avec rhinite allergique était plus élevée que chez les enfants asthmatiques sans rhinite allergique. La concentration de nNO est étroitement liée au FENO et aux facteurs allergiques tels que les IgE, les éosinophiles dans le sang périphérique. Après 6 mois de traitement prophylactique, le taux de contrôle complet de l'asthme chez les enfants asthmatiques était le plus faible selon les critères GINA de 67,5%, et le plus élevé selon les critères ACT à 96,1%. Les concentrations de FeNO et de nNO dans les voies aériennes ont diminué après la prophylaxie de l'asthme et de la rhinite allergique. Les paramètres des éosinophiles sanguins, des IgE sanguins, de la FeNO et de la nNO sont des marqueurs inflammatoires qui aident à classer le phénotype de l'asthme, à prédire la gravité de l'asthme et à prédire/s'attendre à une réponse au traitement par corticostéroïdes.
MOTS CLÉS: Asthme; Rhinite allergique; Monoxide d’azôte; NO; FeNO; Nasal NO.
INTRODUCTION
Asthma is a diverse disease. Every year, the Global Asthma Program (GINA) updates its definitions, investigations in Asthma as well as treatment and prevention protocols.
GINA 2020 [1] defines Asthma as a heterogeneous disease, often characterized by chronic inflammation of the airways. It is defined by a patient history of respiratory symptoms such as wheezing, tachypnea, chest tightness, and cough that vary in duration and intensity, along with fluctuating expiratory airflow limitation.
The presentation of clinical symptoms and the severity of the disease varies from patient to patient, demonstrating the heterogeneity of the disease and making it difficult to diagnose, predict and treat asthma. especially in children.
Asthma and allergic rhinitis are the most common chronic airway diseases in children. According to the Association of Allergic Rhinitis and its Impact on Asthma - ARIA (Allergic Rhinitis and its Impact on Asthma), the rate of bronchial asthma with allergic rhinitis accounts for 80%. In asthmatic patients with allergic rhinitis, studies have confirmed the important role of allergic rhinitis control in asthma control outcomes [2]. Studies show that Allergic Rhinitis worsens Asthma and that treating Allergic Rhinitis improves asthma symptoms. According to Thomas et al., Allergic rhinitis doubles the frequency of hospitalization and increases the number of visits in 1 year of asthma patients (4.3 times compared with 3.3 times) [3].
Asthma control is the primary goal of asthma prophylaxis. There are different methods for assessing asthma control. Until now, we often use the Asthma control test (ACT) to assess asthma control because of its convenience and ease of application on both children and adults. However, this toolkit only assessed asthma symptoms and did not assess the impact of comorbidities on asthma control, especially Allergic Rhinitis [4].
In Vietnam, studies on the value of the CARATkids questionnaire as well as the correlation of this questionnaire with the concentration of NO in the airways on asthma patients are still few, especially in children.
Asthma with Allergic Rhinitis. With the expectation of evaluating the correlation between NO concentration and asthma control status, we conducted the topic "Study on asthma control status in children with bronchial asthma with allergic rhinitis".
METHODS
Objects
154 patients aged 6-15 years were diagnosed with bronchial asthma and were divided into 2 groups: Group 1 was the group of children with asthma with co-morbidities of atopic dermatitis, and Group 2 was a reference group of 30 children with asthma management but no associated allergic rhinitis.
The method for research
Step 1: Select patients to study (time T0)
Patients who come to the National Children's Hospital clinic are asked about their illness, clinical examination, and tests to confirm the diagnosis of bronchial asthma (with and without associated allergic rhinitis)., After being diagnosed with bronchial asthma, patients with or without allergic rhinitis aged 6-15 years old were invited to participate in the study. Children aged 6-15 years with no nasal symptoms, no personal and family history of allergic diseases who attended hospital physical examination were invited to the healthy reference group.
Step 2: Treat and evaluate control of asthma and allergic rhinitis.
Patients with asthma without asthma have their asthma controlled according to GINA 2016 guidelines [5]
- Asthma stage 2: use low dose ICS in combination with SABA when needed.
- Asthma stage 3: moderate dose ICS plus SABA as needed.
ASTHMA patients with V.M. were controlled for asthma according to GINA 2016 and controlled for allergic rhinitis according to ARIA 2008 guidelines [2]
- Intermittent VM - mild: oral antihistamine when symptomatic.
- Intermittent VM – moderate/severe: treat with LTRAs, if necessary, oral antihistamines can be added.
- Persistent - mild VM: treat with LTRAs; if necessary, oral H1 antihistamines can be added. Choose low-dose intranasal corticosteroids if the child has previously had a poor response to H1 antihistamines and LTRAs.
- Persistent - moderate/severe VM: Treat with LTRAs in combination with low-dose intranasal corticosteroids or treat with high-dose nasal data. corticosteroids. If symptoms are severe, add an oral H1 antihistamine at the start of treatment.
- If the nose is blocked, you can add a decongestant spray (xylometazoline hydrochloride 0.05%).
Step 3: Re-examination, assessment of asthma control - allergic rhinitis and adjustment of preventive medicine.
- Time of follow-up: after 1 month of prophylaxis (T1), after 3 months of prophylaxis (T3), and after 6 months of prophylaxis (T6).
- Clinical examination, check treatment adherence, and spray method.
- Measure FeNO, nNO and CNH.
- Interview the asthma control panel according to GINA, ACT and CARAT kids (for children with asthma).
- Adjust asthma prophylaxis according to GINA 2016 guidelines [5].
Step 4: Input and process.
RESULTS
Characteristics of nasal nitric oxide in children with bronchial asthma with allergic rhinitis
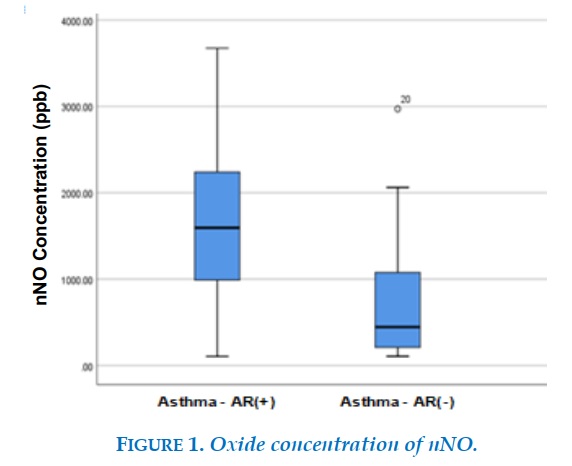
The concentration of nNO in children with bronchial asthma with allergic rhinitis was 1594.5 (104 - 3674) ppb (1604 ± 77ppb) higher than that in the group of bronchial asthma without allergic rhinitis at 444.5 (105 - 2971) ppb (693 ± 122ppb), the difference is statistically significant with p <0.001. The concentration of nNO in the healthy group of children was 1055 (149 - 2090) ppb, lower than the asthma group with allergic rhinitis and higher than the asthmatic group without allergic rhinitis.
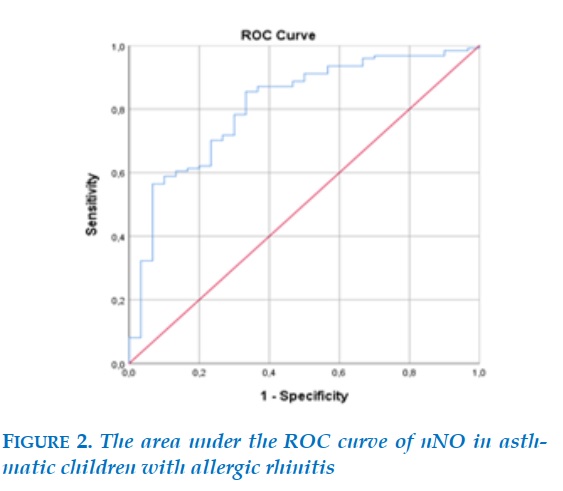
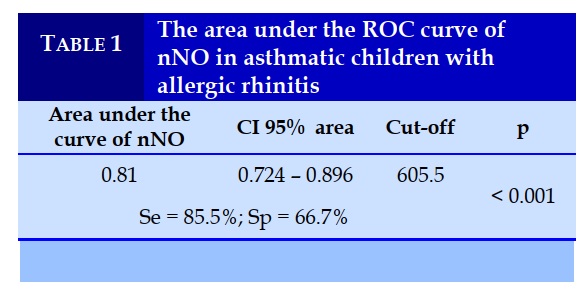
The diagnostic value of allergic rhinitis in patients with bronchial asthma with the area under the ROC curve of nNO is 0.81; with the threshold nNO = 605.5 ppb, the sensitivity is 85.5%, the specificity is 66.7%, p < 0.001.
Change in ICS dose during asthma treatment in asthmatic children with allergic rhinitis
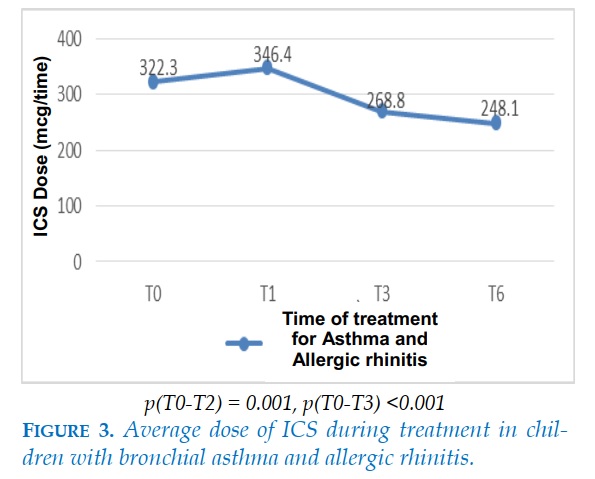
The average dose of ICS at the beginning of treatment of the asthmatic group with allergic rhinitis was 322 ± 124 mcg, slightly increased after 1 month of treatment. The ICS dose was then tapered at the 3rd and 6th months. After 6 months of treatment, the mean ICS dose was 248.1 mcg. The difference was statistically significant with p<0.05.
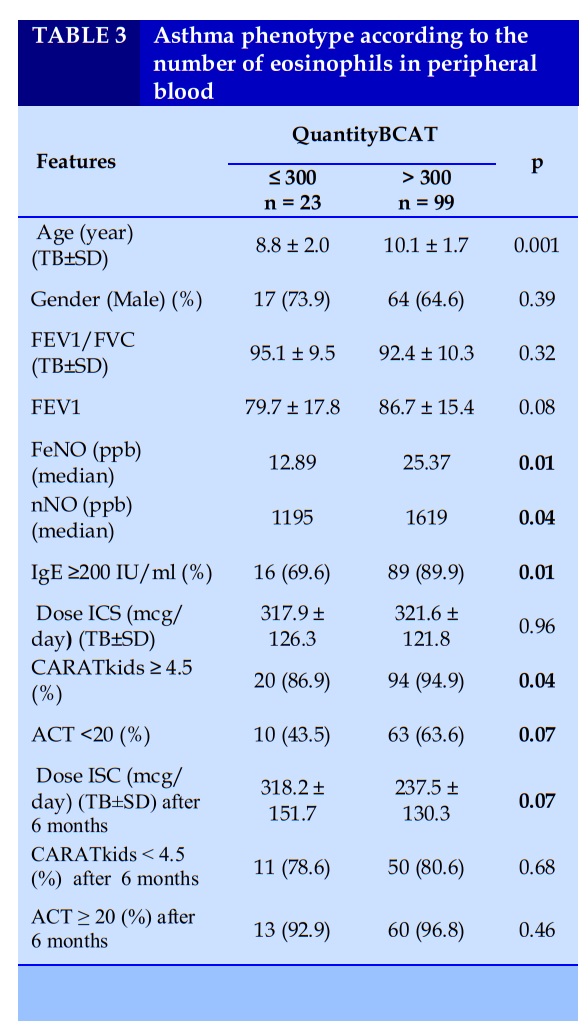
Classification of asthma phenotypes in asthmatic children with allergic rhinitis according to the number of eosinophils in peripheral blood.
Classification of asthma phenotype according to the ratio of BCAT in peripheral blood was divided into two groups, group with BCAT ≤ 300 and group with BCAT > 300. The group with BCAT > 300 had mean age, FeNO, nNO and the rate of total IgE in blood ≥200 IU/ml were higher than the group with BCAT ≤ 300; the difference was statistically significant with p<0.05.
Before prevention, the rate of poorly controlled asthma according to CARAT kids was higher in the group with BCAT > 300 than in the group with BCAT ≤ 300. The dose of ICS after 6 months of asthma control in the BCAT ≤ 300 group was higher than that in the BCAT > group > 300; however, the difference was not statistically significant.
There was no difference in asthma prevention outcomes between the two groups after 6 months of treatment.
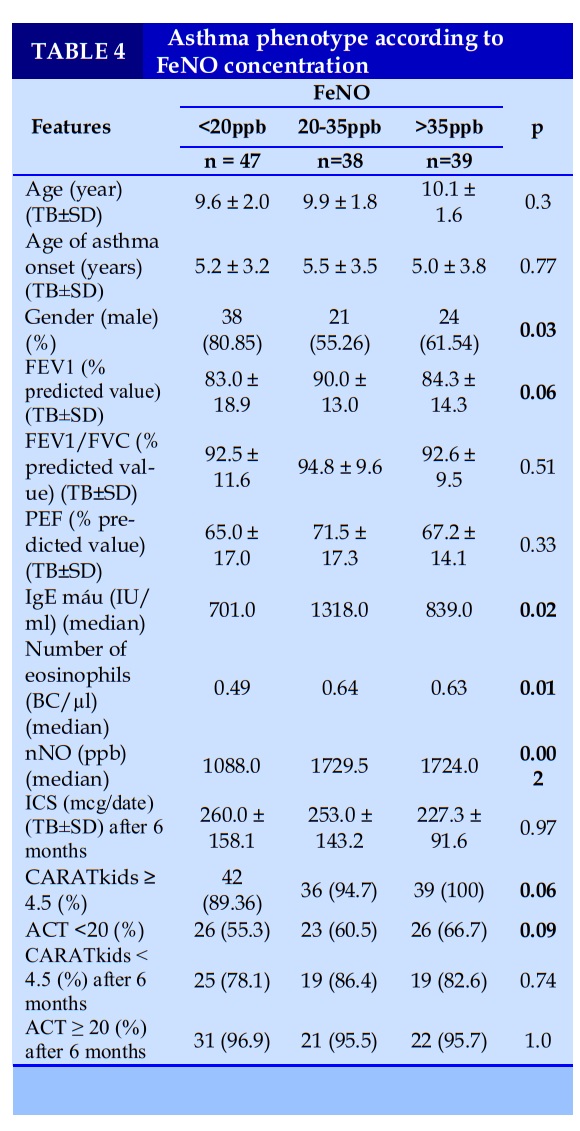
Grouping of asthma phenotypes in children with bronchial asthma with allergic rhinitis according to FeNO concentration.
FeNO was divided into 3 groups: FeNO did not increase (<20ppb), FeNO increased (20-35ppb) and FeNO increased (>35ppb). Classification of asthma phenotypes by FeNO concentration showed that the group with low FeNO had blood IgE concentration, low eosinophil count, and low nNO concentration.
Before treatment, the rate of poor asthma control according to ACT and CARATkids was highest in the elevated FeNO group (>35ppb) and lowest in the non-increased FeNO group (<20ppb), but the difference was not statistically significant with p>0.05. There was no difference in ICS dose and asthma control rate according to ACT and CARATkids in 3 groups after 6 months of treatment (Table 3).
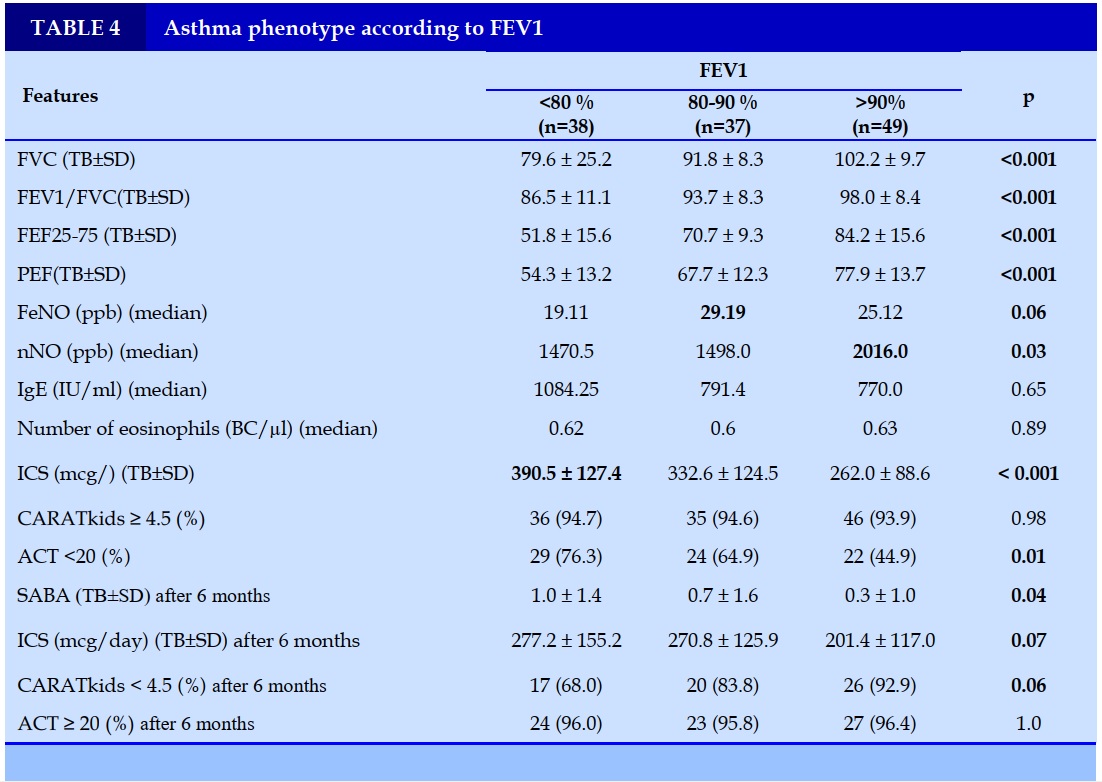
FEV1 was divided into 3 groups, a group with normal FEV1 (>90%), a group with slightly decreased FEV1 (80-90%), and a group with clearly reduced FEV1 (<80%). The group with clearly reduced FEV1 had the lowest FeNO and nNO. At the same time, this group has a higher need for ICS than the other 2 groups and the average number of SABA relievers is 1.0 ± 1.4 times, higher than the group with normal FEV1. Before treatment, the rate of poorly controlled asthma according to ACT in the group with clearly reduced FEV1 (<80%) was higher than in the other two groups. After treatment, the group with a marked decrease in FEV1 (<80%) had a higher dose of ICS, a higher average number of SABA use in a month, and a higher rate of poor asthma control in terms of CARATkids compared to the other two groups (Table 4).
DISCUSSION
nNO concentrations in children
In our study, the value of bronchial asthma with allergic rhinitis was 1594.5 (104 - 3674) ppb higher than the group of children with asthma without allergic rhinitis was 444.5 (105 - 2971). ) ppb and healthy children 1055 (149 – 2090) ppb.
The results of our study are similar to the effects of other studies around the world. According to Sabina et al (2020), a study on 179 subjects including 25 healthy children, 47 children with allergic rhinitis, 49 children with asthma without allergic rhinitis and 58 children with asthma with allergic rhinitis showed that nNO levels in the patient patients with allergic rhinitis were (2322.3 ± 447.24 ppb) and in patients with allergic rhinitis-asthma (2397.3 ± 423.25 ppb), statistically significantly higher than children with asthma alone and in the control group (nNO concentrations were 1017.4 ± 396.85 and 836.2 ± 333.47 ppb, respectively) [6]. However, this result is higher than that of other authors globally, which can be explained by differences in race, climate, environment, and allergy status.
Evaluation of asthma control and allergic rhinitis according to exhaled nitric oxide concentration
One of the pathogenesis of asthma is eosinophilia-associated chronic airway inflammation, which is fundamental for asthma treatment and prevention. FeNO below 20 ppb is the recommended threshold in ATS monitoring of asthma control [7]. In our study, the rate of asthma with reasonable control in terms of FeNO after 1 month, 3 months and 6 months of treatment was 61.8%, 78.6% and 76.6% respectively, higher than before treatment. 37.9%. Before treatment, FeNO levels of children with ASTHMA higher than 20ppb all decreased below 20 ppb at follow-up time after 1 month, 3 months, 6 months and lower than at the first visit (p<0.05). When treated with ICS, airway inflammation improved, clinical symptoms decreased, and FeNO levels decreased.
Asthma phenotype according to the ratio of eosinophils in peripheral blood
Based on the isolation of airway inflammatory cell types, the pathophysiological phenotype of asthma is divided into 4 types: eosinophilic asthma (EA), neutropenic asthma (NA), and mixed asthma. Increased eosinophils and neutrophils (MGA), asthma did not increase the number of cells in the airways (PGA). Under current conditions, the study has not isolated inflammatory cells in the airways, so blood eosinophils are an inflammatory marker to help classify asthma phenotypes. According to the ratio of BCAT in peripheral blood, classification of asthma phenotype was divided into two groups, group with BCAT < 300 and group with BCAT ≥ 300. FeNO, nNO concentration and percentage of patients with total blood IgE ≥ 200 IU /ml in the group with BCAT ≥ 300 were higher than those with BCAT < 300. The difference was statistically significant with p<0.05. Before prevention, the rate of poorly controlled asthma according to CARAT kids was higher in the group with BCAT > 300 than in the group with BCAT ≤ 300. The dose of ICS after 6 months of asthma control in the BCAT ≤ 300 group was higher than that in the BCAT > group > 300. However, the difference was not statistically significant. There was no difference in asthma prevention outcomes between the two groups after 6 months of treatment. In this study, the level of BCAT increase only reflected the level of allergy status, the status of asthma control before treatment, did not find a relationship between the level of BCAT increase with the dose of ICS and the results of asthma control after treatment.
The blood eosinophil count is an inflammatory marker that can help clinicians classify asthma phenotypes and predict treatment response in the general asthma population. However, in the allergic asthma group, the change in BCAT only reflected the degree of inflammation.
Asthma phenotype according to FeNO
We chose the recommended thresholds of FeNO ATS as unchanged FeNO (<20ppb), moderately increased FeNO (20-35ppb), and elevated FeNO (>35ppb) to classify asthma phenotypes. The results showed that the low FeNO group had blood IgE concentration, low eosinophil count, and low nNO concentration. Monitoring control of asthma and allergic rhinitis after 6 months showed that the asthma group with FeNO < 20ppb had a higher need for ICS and the rate of complete asthma control according to CARATkids was lower than the other 2 groups, but the difference There was no statistical significance with p>0.05.
In studies / worldwide, the authors used different clinical and laboratory characteristics such as allergic status, blood eosinophils, airways, blood IgE levels, respiratory function. and FeNO concentration to classify asthma phenotypes to choose the appropriate asthma treatment regimen and treatment prognosis. In children with bronchial asthma, asthmatic children with FeNO concentration <20 ppb are common in overweight and obese children. This is also the group with a high proportion of children with uncontrolled asthma, low respiratory function, and IgE concentration; This is a group of early-onset asthma, with a non-eosinophilic asthma phenotype. The asthmatic children with FeNO ≥35 ppb often had late onset of asthma, a high rate of exposure to tobacco smoke, atopy, good respiratory function, and high IgE concentration, this is the phenotype of leukocytosis. Eosinophils have not been treated prophylactically. This group responded better to ICS than the non-eosinophilic asthma group.
In 2014, Just conducted a study on 125 asthmatic children with an average age of 8.9 years [8]. The authors based on the results of skin prick tests, respiratory function, blood IgE levels, FeNO to evaluate allergic properties.
Asthma phenotype according to respiratory function
Spirometry is a method used to evaluate respiratory function commonly used to diagnose and monitor respiratory diseases in adults and children. The respiratory function helps diagnose asthma with a specificity of 100% through the index of FEV1, FEV1/FVC, and more recently, the index of FEF25-75% [9]. Children with ASTHMA have lower respiratory function than healthy children. Historically, FEV1 has been used to assess asthma severity.
In our study, FEV1 was divided into 3 groups, a group with normal FEV1 (>90%), a group with slightly decreased FEV1 (80-90%), and a group with clearly reduced FEV1 (<80%). The group with a marked decrease in FEV1 had lower respiratory function than the other two groups. This result shows that the indices of respiratory function are closely related and help distinguish the severity of asthma [10].
CONFLICT OF INTERESTS
Non.
REFERENCE
1. (GINA), G.I.f.A., Global strategy for asthma management and prevention (2020 update). 2020.
2. Bousquet, J. and e. al, Allergic rhinitis and its impact on asthma (ARIA) 2008. Allergy 2008. 63: p. 8-160.
3. Thomas, M. and e. al, Asthma-related health care resource use among asthmatic children with and without concomitant allergic rhinitis. Pediatrics 2005. 115(1): p. 129-134.
4. Schatz, M., et al., Asthma Control Test: reliability, validity, and responsiveness in patients not previously followed by asthma specialists. J Allergy Clin Immunol, 2006. 117(3): p. 549-56.
5. (GINA), G.I.f.A., Pocket guide for asthma management and prevention for adults and children older than 5 years. 2016.
6. Galiniak, S., et al., Nasal nitric oxide in upper airways in children with asthma and allergic rhinitis. Advances in Medical Sciences, 2020. 65(1): p. 127-133.
7. Society, A.T. and E.R. Society, ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med, 2005. 171(8): p. 912-30.135.
8. Just, J., et al., Childhood allergic asthma is not a single phenotype. 2014. 164(4): p. 815-820.
9. Lawand, S., V. Budihal, and M.J.J.o.N.R. Karanjkar. Assessment of spirometry parameters in suspected asthmatic children-A clinical study. 2020. 21(3): 41-44.
10. Smith, A.D., et al., Diagnosing asthma: comparisons between exhaled nitric oxide measurements and conventional tests. 2004. 169(4): p. 473-478.
FIGURES - TABLES
REFERENCE
1. (GINA), G.I.f.A., Global strategy for asthma management and prevention (2020 update). 2020.
2. Bousquet, J. and e. al, Allergic rhinitis and its impact on asthma (ARIA) 2008. Allergy 2008. 63: p. 8-160.
3. Thomas, M. and e. al, Asthma-related health care resource use among asthmatic children with and without concomitant allergic rhinitis. Pediatrics 2005. 115(1): p. 129-134.
4. Schatz, M., et al., Asthma Control Test: reliability, validity, and responsiveness in patients not previously followed by asthma specialists. J Allergy Clin Immunol, 2006. 117(3): p. 549-56.
5. (GINA), G.I.f.A., Pocket guide for asthma management and prevention for adults and children older than 5 years. 2016.
6. Galiniak, S., et al., Nasal nitric oxide in upper airways in children with asthma and allergic rhinitis. Advances in Medical Sciences, 2020. 65(1): p. 127-133.
7. Society, A.T. and E.R. Society, ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med, 2005. 171(8): p. 912-30.135.
8. Just, J., et al., Childhood allergic asthma is not a single phenotype. 2014. 164(4): p. 815-820.
9. Lawand, S., V. Budihal, and M.J.J.o.N.R. Karanjkar. Assessment of spirometry parameters in suspected asthmatic children-A clinical study. 2020. 21(3): 41-44.
10. Smith, A.D., et al., Diagnosing asthma: comparisons between exhaled nitric oxide measurements and conventional tests. 2004. 169(4): p. 473-478.
ARTICLE INFO
DOI: 10.12699/jfvpulm.12.37.2021.13
Conflict of Interest
Non
Date of manuscript receiving
15/6/2020
Date of publication after correction
15/7/2021
Article citation
Nguyen-Tran-Ngoc H., Nguyen-Thi-Dieu Th., Tran-Van D., Luong-Cao D., Duong-Quy S.. Study of asthma control status in children with bronchial asthma and allergic rhinitis. J Func Vent Pulm 2021;37(12):7-12