
 English
English
 French
French
A rare case of alpha1-antitrypsin deficiency: Revelation by pulmonary tuberculosis
Un cas rare de déficit en alpha1-antitrypsine: Révélation par tuberculose pulmonaire
A. Benali, H. El Ouazzani
Service de Pneumologie de l’Hôpital Militaire d’Instruction Mohamed V
BP 10045 Rabat. Maroc
Corresponding author
Anass Benali
résidence Ibn Al Arabi Hay Ibn Rochd Temara Maroc BP 12000.
E-mail: anass-ab@hotmail.fr
DOI: 10.12699/jfvpulm.12.37.2021.66
ABSTRACT
Alpha1-antitrypsin, the main inhibitor of neutrophil elastase is supposed to limit the aggression of tissues by proteases at the site of inflammation or infection and participate in maintaining tissue integrity; any quantitative or qualitative modification of this glycoprotein causes an imbalance in the protease / antiprotease balance, responsible for increased destruction of the connective matrix of the lung. The radioclinical pictures of alpha1-antitrypsin deficiency are variable depending on the phenotype. The progression of the disease is favored by several factors, mainly smoking and recurrent respiratory infections representing the main risk factors for the development of pulmonary emphysema. We report a rare case of diagnosis of alpha1-antitrypsin deficiency in a young 39 years old with pulmonary tuberculosis.
KEYWORDS: Deficiency; Alpha1-antitrypsin; Emphysema; Tuberculosis
RÉSUMÉ
L'alpha1-antitrypsine, principal inhibiteur de l'élastase des neutrophiles est censée limiter l'agression des tissus par les protéases au site d'inflammation ou d'infection et participer au maintien de l'intégrité tissulaire ; toute modification quantitative ou qualitative de cette glycoprotéine provoque un déséquilibre de la balance protéase/antiprotéase, responsable d'une destruction accrue de la matrice conjonctive du poumon. Les images radiocliniques du déficit en alpha1-antitrypsine sont variables selon le phénotype. La progression de la maladie est favorisée par plusieurs facteurs, principalement le tabagisme et les infections respiratoires récurrentes représentant les principaux facteurs de risque de développement de l'emphysème pulmonaire. Nous rapportons un cas rare de diagnostic de déficit en alpha1-antitrypsine chez un jeune de 39 ans atteint de tuberculose pulmonaire.
MOTS CLÉS: Carence; Alpha1-antitrypsine; Emphysème; Tuberculose
INTRODUCTION
α1-Antitrypsin deficiency is an autosomal recessive disorder caused by mutation in the SERPINA1 gene, this mutation causes a modification in the conformation of the protein, thus responsible for the decrease in the serum level of α1-Antitrypsin and / or the loss of its function, the consequence of which is mainly represented by the early appearance of emphysema and more rarely by cirrhosis [1].
We report a clinical case of pulmonary emphysema related to a deficiency in α1-antitrypsin revealed by pulmonary tuberculosis in a young 39 years old.
OBSERVATION
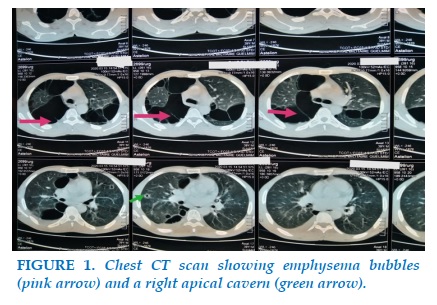
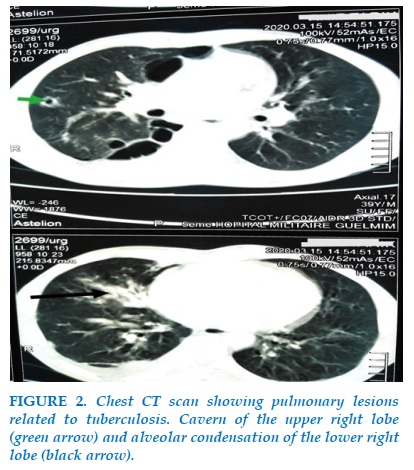
Mr R.T, 39 years old, smokes for 16 pack-years, not followed for a chronic pathology, from a smoking father being treated for chronic obstructive pulmonary disease (COPD), admitted to the pulmonology department for moderately abundant hemoptysis evolving for 15 days in the context of deterioration of general condition, night sweats and fever. In addition, he reports dyspnea that has gradually worsened over the past 2 years, associated with nocturnal episodes of wheezing and also occurring after physical exertion. The biological assessment shows an increase in the reactive C protein to 66 mg / l, the hepatic assessment is normal. Tests for Mycobacterium tuberculosis in sputum are positive on direct examination. The chest CT scan with injection of iodinated contrast product reveals two categories of lesions: the first is related to the pulmonary infection, it is a cavern of the ventral segment of the upper right lobe associated with alveolar condensation of the right lower lobe and the second represents emphysema bubbles involving both lungs and predominantly in the upper lobes (Figures 1, 2). The discovery of pulmonary emphysema bubbles in a 39-year-old smoker presenting chronic respiratory functional signs and having a history of COPD in the family, led to the suspicion of an α1-antitrypsin deficiency confirmed by his serum dosage showing a low rate of 0.5 g / l.
The therapeutic management started is divided into 3 parts: antibacillaries according to the 2 RHZE / 4 RH protocol, smoking cessation and short-acting bronchodilators in the event of signs and long-acting as background treatment and the last part is the transfer of the patient to the university hospital to carry out a family survey, phenotyping and to suggest possible specific treatments for this rare genetic disorder.
DISCUSSION
Alpha-1 antitrypsin deficiency should be suspected in the first place if chronic respiratory signs associated with emphysema lesions occur at an early age, regardless of their form or distribution. In our observation we studied a rare case of alpha-1 antitrypsin deficiency revealed by pulmonary tuberculosis, in a young patient with respiratory signs dominated by dyspnea, and pulmonary lesions predominant in the upper lobes, the confirmation of the diagnosis is ensured by serum protein dosage. Indeed, α1-antitrypsin is a protease inhibitor synthesized by the liver, protects lung alveolar tissues from destruction by neutrophil elastase. Prospective studies in Sweden have shown that only 10% of patients develop clinically significant liver disease by their fourth decade, indicating that other genetic traits or environmental factors likely influence the development of liver disease. Emphysema is not typically observed in children but an increased risk for developing asthma is reported. Cigarette smoking promotes development of lung disease [2, 3].
α1-Antitrypsin is highly specialized in the capture and inactivation of serine proteases. Its spectrum of activity is extended to cathepsin G, proteinase 3 and neutrophil elastase for which it has the strongest affinity. Its main function is to neutralize, in the event of bacterial infection or inflammation, elastase released in excess by PNNs and to protect the extracellular matrix from proteolytic degradation [4]. α1-Antitrypsin deficiency is the only known genetic cause of emphysema and is found in 1- 2% of all individuals with chronic obstructive pulmonary disease (COPD); COPD will be the third commonest cause of death worldwide by 2020. The only treatment directly targeting the underlying pathobiology of the lung disease is α1-Antitrypsin augmentation therapy [2]. The main mutation observed in northern Europe is the Z form, it is the most common and represents 95% of clinically recognized deficits [5]. In a French study exploring the characteristics of emphysematous patients with α1-Antitrypsin deficiency of the PiZ phenotype, the age of onset of the disease was found to be between the 3rd and 4th decade, dyspnea represents the most frequent symptom found in 95% of patients, functional respiratory exploration reveals that two thirds of patients have severe to very severe bronchial obstruction, the frequency of exacerbations is all the more important as the respiratory function is impaired and whose causes are mainly infections [6], in our case it is pulmonary tuberculosis allowing the revelation of this genetic disorder which is not described in the literature. The lack of α1-Antitrypsin predisposes the Z homozygote to early onset panlobular basal emphysema [7]. People with the PiMZ phenotype are at increased risk for COPD, as a result of interactions between genetic factors, smoking and the environment. In the event of smoking, discreet spirometric abnormalities appear at a late age but they can be accelerated by recurrent environmental attacks, moreover, there does not seem to be any hepatic complication in heterozygote subjects [8,9]. The SZ phenotype does not pose a risk of emphysema in non-smokers but smoking exposes this risk significantly, in terms of imaging, the lesions seem less severe than for the homozygous PiZ phenotype with a distribution apical more frequent [10]. The therapeutic management consists essentially of a non-specific medical treatment almost similar to that of COPD and which mainly comprises inhaled therapies, smoking cessation and the management of exacerbations, in addition to a substitution treatment indicated in the severe forms and which targets to restore serum alpha-1 antitrypsin concentrations in order to prevent or slow the degradation of lung tissue; currently treatment is based exclusively on weekly or monthly intravenous administration of Alpha-1 antitrypsin (increases) whose effectiveness has been demonstrated [11,12].
CONCLUSION
Alpha-1 antitrypsin deficiency is a rare genetic disorder, the clinical, functional and radiological presentation varies depending on the severity of the deficiency and therefore according to the phenotype, which also conditions the treatment. Disclosure during the diagnosis of pulmonary tuberculosis is a very rare situation.
CONFLICT OF INTERESTS
The authors declare that they have no conflicts of interest in relation to this article.
REFERENCES
1. Crystal R. Alpha-1 antitrypsin deficiency, emphysema and liver disease. J Clin Invest 1990;85:1343–52.
2. Lomas DA, Hurst JR, Gooptu B: Update on alpha-1 antitrypsin deficiency: new therapies, J Hepatol.2016; 65(2):413–424.
3. Nelson D, Teckman J, Di Bisceglie A, et al: Diagnosis and management of patients with α1-antitrypsin (A1AT) deficiency, Clin Gastroenterol Hepatol .2012;10:575–580.
4. Churg A, Wang X, Wang RD, Meixner SC, Pryzdial EL, Wright JL. Alpha-1 antitrypsin suppresses TNF-_ and MMP12 production by cigarette smoke-stimulated macrophages. Am J Respir Cell Mol Biol 2007;37:144–51.
5. Zerimech F, Hennache G, Bellon F, Barouh G, Laffite JJ, Porchet N,et al. Evaluation of a new sebia isoelectro focusing kit for alpha-1 antitrypsin phenotyping with the Hydrasis System. Clin Chem Lab Med 2008;46:260–3.
6. Thabut G, Mornex JF, Cuvelier A, Padrazzi B, Pison C, Neukirch F, et al Caractéristiques des patients inclus dans la cohorte française de patients emphysémateux déficients en alpha-1 AT. Rev Mal Respir 2008;25:1115–22.
7. Eriksson S. Studies in α1-Antitrypsin deficiency. Acta Med Scand 1965;432:1–85.
8. ATS/ERS statement: standards for the diagnosis and management of individuals with alpha-1 antitrypsin deficiency. Am J Respir Crit Care Med 2003;168:818-900.
9. Perlmutter DH. Alpha-1 antitrypsin deficiency: diagnosis and treatment. Clin Liver Dis 2004;8:839–59.
10. Holme J, Stockley RA. CT scan appearance, densitometry, and health status in protease inhibitor SZ alpha-1-antitrypsin deficiency. Chest 2009;136:1284–90.
11. Wewers MD, Casolaro MA, Sellers SE, Swayze SC, McPhaule K. Replacement therapy for alpha-1-antitrypsin deficiency associated with emphysema. N Engl J Med 1987;316:1055–62.
12. Dirksen A, Dijkman JH, Madsen F, Stoel B, Hutchinson DC. A randomized clinical trial of alpha-1-antitrypsin augmentation therapy. Am J Respir Crit Care Med 1999;160:1468–72.
FIGURES
REFERENCES
1. Crystal R. Alpha-1 antitrypsin deficiency, emphysema and liver disease. J Clin Invest 1990;85:1343–52.
2. Lomas DA, Hurst JR, Gooptu B: Update on alpha-1 antitrypsin deficiency: new therapies, J Hepatol.2016; 65(2):413–424.
3. Nelson D, Teckman J, Di Bisceglie A, et al: Diagnosis and management of patients with α1-antitrypsin (A1AT) deficiency, Clin Gastroenterol Hepatol .2012;10:575–580.
4. Churg A, Wang X, Wang RD, Meixner SC, Pryzdial EL, Wright JL. Alpha-1 antitrypsin suppresses TNF-_ and MMP12 production by cigarette smoke-stimulated macrophages. Am J Respir Cell Mol Biol 2007;37:144–51.
5. Zerimech F, Hennache G, Bellon F, Barouh G, Laffite JJ, Porchet N,et al. Evaluation of a new sebia isoelectro focusing kit for alpha-1 antitrypsin phenotyping with the Hydrasis System. Clin Chem Lab Med 2008;46:260–3.
6. Thabut G, Mornex JF, Cuvelier A, Padrazzi B, Pison C, Neukirch F, et al Caractéristiques des patients inclus dans la cohorte française de patients emphysémateux déficients en alpha-1 AT. Rev Mal Respir 2008;25:1115–22.
7. Eriksson S. Studies in α1-Antitrypsin deficiency. Acta Med Scand 1965;432:1–85.
8. ATS/ERS statement: standards for the diagnosis and management of individuals with alpha-1 antitrypsin deficiency. Am J Respir Crit Care Med 2003;168:818-900.
9. Perlmutter DH. Alpha-1 antitrypsin deficiency: diagnosis and treatment. Clin Liver Dis 2004;8:839–59.
10. Holme J, Stockley RA. CT scan appearance, densitometry, and health status in protease inhibitor SZ alpha-1-antitrypsin deficiency. Chest 2009;136:1284–90.
11. Wewers MD, Casolaro MA, Sellers SE, Swayze SC, McPhaule K. Replacement therapy for alpha-1-antitrypsin deficiency associated with emphysema. N Engl J Med 1987;316:1055–62.
12. Dirksen A, Dijkman JH, Madsen F, Stoel B, Hutchinson DC. A randomized clinical trial of alpha-1-antitrypsin augmentation therapy. Am J Respir Crit Care Med 1999;160:1468–72.
ARTICLE INFO
DOI: 10.12699/jfvpulm.12.37.2021.66
Conflict of Interest
Non
Date of manuscript receiving
15/6/2020
Date of publication after correction
15/7/2021
Article citation
Benali A., El Ouazzani H.. A rare case of alpha1-antitrypsin deficiency: Revelation by pulmonary tuberculosis. J Func Vent Pulm 2021;37(12):66-68