
 English
English
 French
French
Early diagnosis of Interstitial Lung Disease without open lung biopsy
Diagnostic précoce de la maladie pulmonaire interstitielle sans biopsie pulmonaire ouverte
M. Hidayath Hussain1, W. Noordin2
1: Mohammed Hidayath Hussain. Department of Pulmonology. Shadan Institute of Medical Sciences. Hyderabad, Telangana
2: Wadhvaniya Noordin, Canadian Specialist Hospital Dubai
Corresponding author
Dr. Wadhvaniya Noordin
Canadian Specialist Hospital Dubai
E-mail: drhidayathsims@gmail.com
ABSTRACT
Objective. The primary objective of this study was to diagnose interstitial lung disease in patients with thorough clinical history, physical exam, chest radiograph, high resolution CT scan, pulmonary function tests (without open lung biopsy) for early diagnosis of disease and to reduce the progression of disease.
Methods. Patients with ILD were included in the study. Radiologic, spirometric evaluation was done and the results are statistically analyzed.
Results. Most common form ILD observed was IPF (37.2%) and collagen vascular disease (5.1%). Majority of cases belonged to the age groups 41-50yrs (26%) and 51 -60 yrs (26%). Most of the cases were males (63.7%) and dyspnoea (100%) on exertion and cough (97%) were the most common presenting features. In reference to radiological abnormalities most common pattern observed is honey combing (45.8%) and ground glass (15.2%) & reticular in (13.5%) of patients. 63.6% of cases of IPF and 33 % of RA-ILD showed honey combing features. Associated features like shaggy cardiac borders, elevated domes of diaphragm and lymphadenopathy, pleural reaction were observed in 42.4% of patients. The Zonal distribution of the disease on chest X-ray showed that (6.8%) of patients involve mid and lower zones. Only lower zones were involved in 23.7 % subjects and mid-zone in 1.7%. Involvement of all zones was observed in 64.4 % of cases. Spirometric function was observed in subjects. Restrictive defect was observed in 81.3 % of patients and mixed defect was observed in 18.6 % patients.
Conclusion. HRCT is significantly superior to chest radiography in identifying and determining the correct diagnosis of ILD. A confident clinical diagnosis of IPF can be reliably made in the presence of characteristic HRCT and clinical findings.
KEYWORDS: Interstitial Lung Disease; Diagnosis; Clinical methods
RÉSUMÉ
Objectif. L'objectif principal de cette étude était de diagnostiquer une maladie pulmonaire interstitielle chez des patients ayant des antécédents cliniques approfondis, un examen physique, une radiographie thoracique, une tomodensitométrie à haute résolution, des tests de la fonction pulmonaire (sans biopsie pulmonaire ouverte) pour un diagnostic précoce de la maladie et pour réduire la progression de la maladie.
Méthodes. Les patients atteints de ILD ont été inclus dans l'étude. Une évaluation radiologique et spirométrique a été effectuée et les résultats sont analysés statistiquement.
Résultats. La forme la plus courante d'ILD observée était l'IPF (37,2%) et les maladies vasculaires au collagène (5,1%). La majorité des cas appartenaient aux groupes d'âge de 41 à 50 ans (26%) et de 51 à 60 ans (26%). La plupart des cas étaient des hommes (63,7%) et la dyspnée (100%) à l'effort et la toux (97%) étaient les caractéristiques de présentation les plus courantes. En référence aux anomalies radiologiques, le schéma le plus commun observé est le peignage au miel (45,8%) et le verre dépoli (15,2%) et réticulaire chez (13,5%) des patients. 63,6% des cas d'IPF et 33% de RA-ILD présentaient des caractéristiques de peignage du miel. Des caractéristiques associées comme des bordures cardiaques hirsutes, des dômes élevés de diaphragme et de lymphadénopathie, une réaction pleurale ont été observées chez 42,4% des patients. La distribution zonale de la maladie sur la radiographie pulmonaire a montré que (6,8%) des patients impliquaient des zones médianes et inférieures. Seules les zones inférieures étaient impliquées chez 23,7% des sujets et la zone médiane chez 1,7%. L'implication de toutes les zones a été observée dans 64,4% des cas. La fonction spirométrique a été observée chez les sujets. Un défaut restrictif a été observé chez 81,3% des patients et un défaut mixte a été observé chez 18,6% des patients.
Conclusion. HRCT est significativement supérieure à la radiographie thoracique pour identifier et déterminer le diagnostic correct de l'ILD. Un diagnostic clinique sûr de la FPI peut être établi de manière fiable en présence de HRCT caractéristiques et de résultats cliniques.
MOTS CLÉS: Maladie pulmonaire interstitielle; Diagnostic; Méthodes cliniques
INTRODUCTION
The interstitial lung diseases are a clinically challenging and diverse group of over 150 disorders characterized by varying degrees of fibrosis and inflammation of the lung parenchyma or interstitium. The interstitial lung diseases represent many features in common such as similarities of symptoms, comparable appearance of chest imaging studies consistent alterations in pulmonary physiology and typical histological features. However ILD are difficult to classify because approximately 150 known diseases are characterized by interstitial involvement, either as a primary disease or as a part of multi organ process as may occur in collagen vascular diseases one useful approach is to separate ILD into two groups, those with known or unknown causes, for each ILD there may be an acute phase followed by a phase of chronicity with acute exacerbation. Among ILD of known cause, the largest group includes diseases due to Inhalation of inorganic dusts, organic dusts and various irritant and noxious gases. The number of ILD of unknown cause is also very large. The major ones are Idiopathic Pulmonary Fibrosis, Sarcoidosis, ILD associated with collagen vascular diseases.
When a clinician is confronted with a patient with possible ILD, in addition to a detailed medical history , physical examination and routine lab tests, a rationale approach to a diagnostic evaluation includes noninvasive diagnostic techniques such as pulmonary function tests including exercise stress test and chest imaging. Invasive diagnostic procedures such as bronchoalveolar lavage, transbronchial lung biopsy and open lung biopsy are taken up when a specific cause of ILD remains unclear. Pulmonary function tests are an important means for establishing the presence of ILD. They are also useful for the clinical monitoring of the disease course and to assess the efficacy of treatment. It is observed that a fraction of biopsy proven cases of ILD have normal plain chest films. Hence characteristic pulmonary function and exercise test abnormalities can suggest ILD and provide justification for lung biopsy.
Exercise test is the most sensitive diagnostic test for ILD since in some patients with biopsy proven ILD the physiological responses to exercise are distinctly abnormal, even though base line pulmonary functions including DLCO and arterial blood gases, chest radiograph and HRCT scan are all normal. Exercise response of ILD patients include a progressive widening of A-a O2, elevated physiological dead space, marked reduction in overall exercise tolerance and low oxygen pulse.. Plain radiograph of the chest remains the cornerstone of the basic imaging in ILD's though in early stages the chest films can be normal.
In addition, it is a good means of monitoring progression of disease and response to treatment. It can also provide diagnostic points to possible etiologies.
CT and HRCT scans are more sensitive and can detect abnormalities better than chest radiography. Preliminary studies suggest that when HRCT pattern is combined with clinical and radiological findings, it can have a diagnostic utility. It can also detect areas of nonfibrotic active disease and relatively unaffected areas to guide the site selection for biopsy. Bronchoalveolar lavage is of diagnostic value when the specimen contains infectious agent or neoplastic cell and altered CD 4: CD8 are detected. Open lung biopsy and thoracoscopic guided lung biopsy merit consideration as final diagnostic step in selected patients. Since the exact etiology and pathogenesis of most ILD are unclear, the treatment options are non specific the therapy is aimed at reducing the inflammation so that the fibrosis can be halted and the rate of progression can be slowed down.
The clinical response is variable. Prognosis is excellent in some ILD like hypersensitivity pneumonitis when the noxious stimulus is removed .In chronic fibrotic disorders, generally the prognosis is poor. Almost half of the patients of IPF, experience subjective improvement with corticosteroid therapy and other immunosuppressive agents, but only 20 to 25% demonstrate objective improvement. The PaO2 at rest and during exercise testing is superior to traditional PFT, to monitor physiologic response to therapy. Supplemental oxygen should be given to all patients with resting hypoxemia, as well those with oxy-hemoglobin desaturation with exertion. Heart and lung or single lung transplantation for highly selected patients with end stage pulmonary fibrosis is available. Relentless progression of the disease with eventual respiratory insufficiency is inevitable and the five years survival rate is above 50% .It is to be hoped that, in future major advances will be made in understanding the pathogenesis of the disorder so that novel therapeutic strategies may be invented.
METHOD
Place of study: Shadan Institute of Medical Sciences.
Type of study: This was a Cross Sectional study.
Sample collection: Sample Size: 69.
Sampling methods: Consecutive patients.
Inclusion Criteria
Chest symptoms – cough, shortness of breath with or without wheeze with enquiry of rashes arthralgia.
Radiological appearance of reticular, nodular, reticulo-nodular or honey combing patterns.
Exclusion Criteria. 1-Tuberculosis, 2- Lung fibrosis,3-Firbothorax,4-Lung Cancer.
Statistical methods. The results are statistically analyzed using appropriate statistical software.
RESULTS
Majority of cases belonged to the age groups 41-50yrs (26%) and 51 -60 yrs (26%) and most of the cases were males (63.7%).
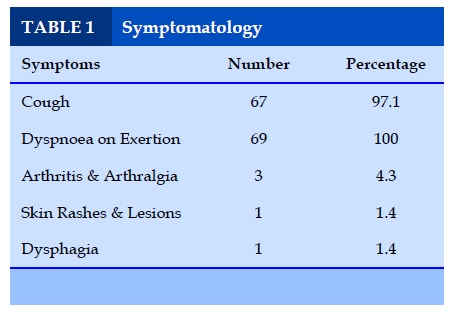
Symptomatology
From the above table it is evident that dyspnoea (100%) on exertion and cough (97%) were the most common presenting features of Interstitial Lung Disease (ILD).
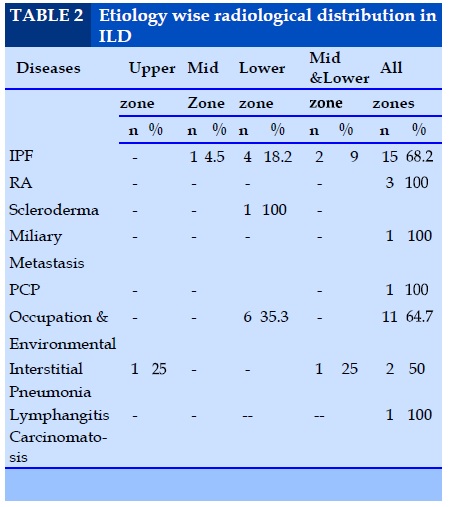
Etiology wise radiological distribution in ILD
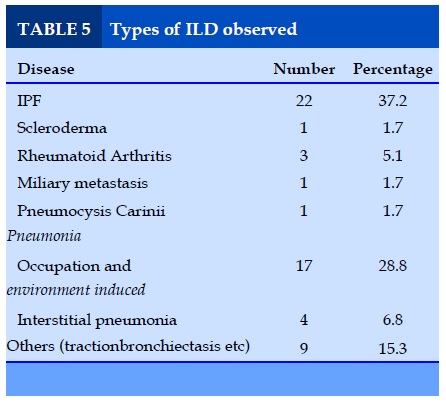
As shown in table 2 in the present study, the most common cause of ILD is IPF accounting for 37.2% of cases followed by 28.8%, occupation & environmental induced diseases.
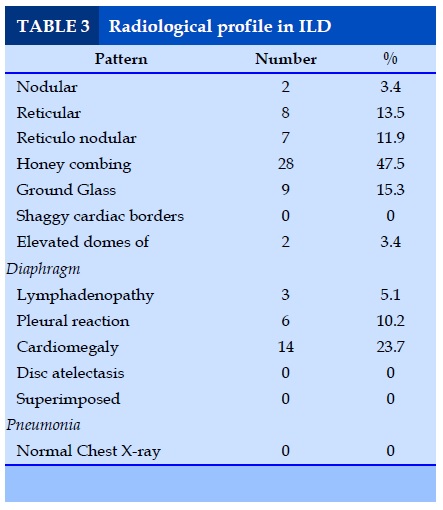
Radiological profile in ILD
In current study the characteristic radiographic appearances observed are the Honey combing (47.5%), Reticulation (13.5%) and Reticulo-nodular (11.8%) patterns. Associated features like shaggy cardiac borders, elevated domes of diaphragm, lymphadenopathy were seen in 32.2% cases. 9.9 % cases of IPF and 0% RA-ILD showed reticulo-nodular features. Honey combing which indicates fibrotic end stage of ILD was seen in 63.6% of IPF and 0 % scleroderma.16.9% of cases showed ground glass appearance.
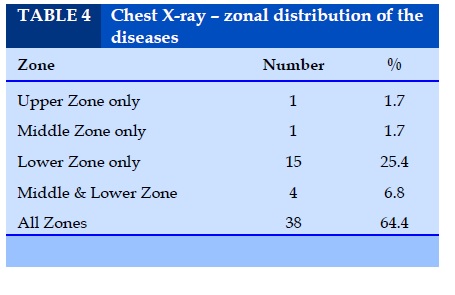
Chest X-Ray – zonal distribution of the diseases
9.9% of IPF showed mid and lower zone involvement and involvement of all zones seen in 68.1 % of cases of IPF. Of the 4 cases of CVD-ILD, 3 cases showed all zone involvement and one case scleroderma showed lower zone involvement.
Types of ILD observed
Predominate disease observed of all the ILD was IPF (37.2%) followed by occupation and environment induced (28.8%) variant.
DISCUSSION
Interstitial lung diseases are heterogeneous group of diseases involving lung interstitium. They have features in common like similarities of symptoms, comparable radiographic appearances, consistent alterations in the pulmonary physiology and typical histological features. In studied during the period from November 2017 - December2018).
Shadan Institute of Medical Sciences, Hyderabad in the Department of Pulmonary Medicine Hyderabad, Telengana. The study group included various etiological forms of interstitial lung diseases such as IPF, ILD due to CVD, military metastasis, PCP and pneumoconiosis etc. In addition to clinical profile, the chief aim was to study the HRCT chest scan appearances. In the present study of the total 69 patients take 59 were proved to be ILD by HRCT scan.
In a study of patients conducted Lim G et al (Korea) the most common cause of ILD was IPF which was followed by CVD – PF and hypersensitivity pneumonia. In present study also the most common cause is IPF accounting for 37.2% of cases followed by 28.8%, occupation &environmental induced diseases.
In the references to the study by Lim GI et al the sex ratio was equal in males & females in IPF, CVD – PF. But the female incidence was greater in sarcoidosis and male incidence in pneumoconiosis. In the present study the males account for majority (54.5%) in IPF. In CVD also males accounted for 100% of cases. Over all, males accounted for majority of the cases in ILD with 62.7 % in the current study. In CVD-PF, the most common cause is Rheumatoid arthritis in Lim GI et al study, in this study also RA are most common causes in CVD-PF. Glazen‘ study of patients on radiographic appearances in ILD described the characteristic radiographic appearances of ILD – the ground glass effect, consolidation, cysts – honey combing pulmonary modules and interstitial thickening. They also included the advantage of HRCT in ILD.
In current study the characteristic radiographic appearances observed are the Honey combing (47.5%) Reticulation (13.5%) Reticulo nodular(11.8%). Associated features like shaggy cardiac borders, elevated domes of diaphragm, lymphadenopathy were seen in 32.2% cases. 9.9 % cases of IPF and 0% RA-ILD showed reticulo- nodular features. Honey combing which indicates fibrotic end stage of ILD was seen in 63.6% of IPF and 0 % scleroderma.16.9% of cases showed ground glass appearance. 9.9% of IPF showed mid and lower zone involvement and involvement of all zones seen in 68.1 % of cases of IPF.
Of the 4 cases of CVD-ILD, 3 cases showed all zone involvement and one case scleroderma showed lower zone involvement. HRCT chest was done in all cases. HRCT is more sensitive than chest roentgenogram in ILD. HRCT showed changes like reticulo-nodular pattern, traction bronchiectasis, and honey combing. Pulmonary function was studied in all patients 76.8 % has restrictive defect, 23.1% of cases had mixed defect. There is no isolated obstructive defect. Detailed history taking is required to identify respiratory risk factors both past and present.
CONCLUSION
HRCT is significantly superior to chest radiography in identifying and determining the correct diagnosis of ILD. The combined information from clinical, laboratory and HRCT findings allows a correct diagnosis to be made in the majority of patients with ILD. In the appropriate clinical setting appearances on the HRCT scan may be sufficiently characteristic to preclude the need for BAL or lung biopsy and histo-pathological examination. Surgical lung biopsy when required should be performed before the initiation of treatment. However some select patients below 50yrs of age, with diffuse shadows, fever, weight loss, and with rapid deterioration may require a trans lung biopsy to rule out malignancy or infective process14 Multiple multi lobe lung biopsies are technically easier by VATS procedure than open lung biopsy. VATS is also associated in the less postoperative pain than open lung biopsy. A confident clinical diagnosis of IPF can be reliably made in the presence of characteristic HRCT and clinical findings. Regarding DLCO this test is used for diagnosis, reduction of DLCO is seen but it’s a non specific finding, Due to the cost factor and the less reliability this test was not done. Role of BAL is ILD is limited but can be used in with acute onset of ILD; such patients can be evaluated for Acute Eosinophillic Pneumonia, Alveolar Hemorrhage and Malignancy.
CONFLICT OF INTEREST
The authors declare any conflict of interest.
REFERENCES
1. Fishman’s pulmonary diseases and disorders. Fourth edition volume 1 page no: 1038-1178.
2. Crofton and Douglas respiratory diseases 5th edition volume 2 page no: 877-891, 1002-1072.
3. Text book of Diagnosis of the Chest by Fraser and Pare’s fourth edition volume III page no : 1421-1621.
4. Cecil Text book of Medicine volume – 1 page no : 396-402.
5. Seminars in Respiratory and critical care medicine on interstitial lung diseases part II, volume 15, by Ganesh Raghu M.D., Guest editor.
6. Text book of medical physiology by Guyton, 10th edition page no : 456-460.
7. Text book of Principles by of internal medicine by Harrisons II volume page no : 1460-1670.
8. New England Journal of Medicine Gross and humming hake 345 (7) : 517.
9. Coultas DB, Zum walt RE, Black WC, Sobonya RE. The epidemiology of Interstitial lung diseases AM J Respiratory critical care medicine 150 : 967-972, 1994.
10. Pathophysiology of activity limitations in patients with Interstitial lung diseases chest 109:1566-1576, 1996.
11. Correlation of Chest roentgenogram with pulmonary function and bronchoalveolar lavage in interstitial lung diseases by Nugent – Kn, Peterson Mu, Jokes H chest 96; 1224-1227, 1989.
12. Padley SPG, Hansell DM, flower CDR, Jenning P. comparative accuracy of HRCT and chest radiograph in the diagnosis chronic diffuse ILD clinical radiology 44 : 222-226, 1991.
13. Raghu G. Cain K, Hammer S. Winter bauer R improved forced vital capacity, DLCo and resting P (A-a) O2 measurements at one year predict longterm survival in idiopathic pulmonary fibrosis AM. Rev. Respiratory Diseases 143:A 57. 1991.
14. Raghu G : Interstitial lung diseases : A diagnostic approach, Are CT scan and lung biopsy indicated in every patient. AM J. respiratory critical care medicine 15:909-914, 1995.
15. Grenier P, chevret S Beigelman C etal : chronic diffuse interstitial lung diseases. Determination of diagnostic value of clinical data, chestradiography and CT with Bayesian Analysis, Radiology 191 : 383-390, 1994.
16. Oxygen improves maximal exercise performance in ILD by Harris – Eze AO, Sridhar G, Clemens RE etal.
17. Robertson HT clinical application of pulmonary function and exercise tests in the management of patients with ILD seminar in Respiratory critical medicine 15:1-9, 1994.
18. Terrif BA, Kwan Sy, Chan – yung MM, Muller NL fibrosing alveolitis, chest radiography and CT as predictors of clinical and functional impairment at follow up in 26 patients. Radiograph 184 : 445-449, 1992.
19. Walters LC, King TE, Schwarz MI etal, a clinical radiographic, and physiologic scoring system for the for the longitudinal assessment of patients with IPF Am revised respiratory diseases 133:97-103, 1986.
20. An analysis of sub maximal exercise response in patients with sarcoidosis and fibrosing alveolitis by spiro SG, Dowdeswell IRG, Clark TGH, Brj Dischest 1981; 75:169-80.
21. Younes M. Interpretation of clinical exercise testing in respiratory diseases clinical chest medicine 1984:5:189-206.
22. Austrian R, Mc clement JH, Renzethi AD etal clinical and physiological features of some types of pulmonary diseases with impairment of alveolar capillary diffuses Am I med 1951;11:667-85.
23. Ohshimo S,Bonella F,Cui A,et al Significance of Bronco-Alveolar Lavage for the Diagnosis of Idiopathic Pulmonary Fibrosis American Journal Of Respiratory and Critical Care Medicine2009 179;1043.
24. BradleyB,Branley HM,Egan JJ et al Interstitial lung disease guidelines The British Thoracic Society in collaboration with Thoracic Society of Australia and New Zealand and Irish thoracic society THORAXD2008;63 Supplenent5:v1.
FIGURES - TABLES
REFERENCES
1. Fishman’s pulmonary diseases and disorders. Fourth edition volume 1 page no: 1038-1178.
2. Crofton and Douglas respiratory diseases 5th edition volume 2 page no: 877-891, 1002-1072.
3. Text book of Diagnosis of the Chest by Fraser and Pare’s fourth edition volume III page no : 1421-1621.
4. Cecil Text book of Medicine volume – 1 page no : 396-402.
5. Seminars in Respiratory and critical care medicine on interstitial lung diseases part II, volume 15, by Ganesh Raghu M.D., Guest editor.
6. Text book of medical physiology by Guyton, 10th edition page no : 456-460.
7. Text book of Principles by of internal medicine by Harrisons II volume page no : 1460-1670.
8. New England Journal of Medicine Gross and humming hake 345 (7) : 517.
9. Coultas DB, Zum walt RE, Black WC, Sobonya RE. The epidemiology of Interstitial lung diseases AM J Respiratory critical care medicine 150 : 967-972, 1994.
10. Pathophysiology of activity limitations in patients with Interstitial lung diseases chest 109:1566-1576, 1996.
11. Correlation of Chest roentgenogram with pulmonary function and bronchoalveolar lavage in interstitial lung diseases by Nugent – Kn, Peterson Mu, Jokes H chest 96; 1224-1227, 1989.
12. Padley SPG, Hansell DM, flower CDR, Jenning P. comparative accuracy of HRCT and chest radiograph in the diagnosis chronic diffuse ILD clinical radiology 44 : 222-226, 1991.
13. Raghu G. Cain K, Hammer S. Winter bauer R improved forced vital capacity, DLCo and resting P (A-a) O2 measurements at one year predict longterm survival in idiopathic pulmonary fibrosis AM. Rev. Respiratory Diseases 143:A 57. 1991.
14. Raghu G : Interstitial lung diseases : A diagnostic approach, Are CT scan and lung biopsy indicated in every patient. AM J. respiratory critical care medicine 15:909-914, 1995.
15. Grenier P, chevret S Beigelman C etal : chronic diffuse interstitial lung diseases. Determination of diagnostic value of clinical data, chestradiography and CT with Bayesian Analysis, Radiology 191 : 383-390, 1994.
16. Oxygen improves maximal exercise performance in ILD by Harris – Eze AO, Sridhar G, Clemens RE etal.
17. Robertson HT clinical application of pulmonary function and exercise tests in the management of patients with ILD seminar in Respiratory critical medicine 15:1-9, 1994.
18. Terrif BA, Kwan Sy, Chan – yung MM, Muller NL fibrosing alveolitis, chest radiography and CT as predictors of clinical and functional impairment at follow up in 26 patients. Radiograph 184 : 445-449, 1992.
19. Walters LC, King TE, Schwarz MI etal, a clinical radiographic, and physiologic scoring system for the for the longitudinal assessment of patients with IPF Am revised respiratory diseases 133:97-103, 1986.
20. An analysis of sub maximal exercise response in patients with sarcoidosis and fibrosing alveolitis by spiro SG, Dowdeswell IRG, Clark TGH, Brj Dischest 1981; 75:169-80.
21. Younes M. Interpretation of clinical exercise testing in respiratory diseases clinical chest medicine 1984:5:189-206.
22. Austrian R, Mc clement JH, Renzethi AD etal clinical and physiological features of some types of pulmonary diseases with impairment of alveolar capillary diffuses Am I med 1951;11:667-85.
23. Ohshimo S,Bonella F,Cui A,et al Significance of Bronco-Alveolar Lavage for the Diagnosis of Idiopathic Pulmonary Fibrosis American Journal Of Respiratory and Critical Care Medicine2009 179;1043.
24. BradleyB,Branley HM,Egan JJ et al Interstitial lung disease guidelines The British Thoracic Society in collaboration with Thoracic Society of Australia and New Zealand and Irish thoracic society THORAXD2008;63 Supplenent5:v1.