
 English
English
 French
French
Clinical process in indications and application of HFNC therapy in patients with severe Covid-19
Processus clinique dans les indications et l'application de la thérapie avec HFNC aux patients atteints de Covid-19 sévère
Duong-Quy Sy1,2, Huynh Truong Anh Duc3, Nguyen Thi Kim Thanh3, Nguyen Quang Tien3, Tran Ngoc Anh Thuy3, Nguyen Van Hoai Nam3, Do Thi Thu Mai3, Nguyen-Van Toi1, Tang-Thi-Thao Tram1, Nguyen-Tuan Anh1, Trinh Du The4, Tran Thai Tuan4, Nguyen Van Tinh3
1: Biomedical Research Center. Lam Dong Medical College. Dalat city, Vietnam
2: Hershey Medical Center. Penn State Medical College. PA, USA
3: Binh Duong General Hospital. Binh Duong province, Vietnam
4: Ninh Thuan General Hospital. Ninh Thuan province, Vietnam
Corresponding author:
Pr. Sy Duong-Quy. Lam Dong Medical College. Dalat city, Vietnam
E-mail: sduongquy.jfvp@gmail.com
ABSTRACT
HFNC (high flow nasal canula) was first introduced into clinical practice in the early 2000s as a non-invasive system for the management of dyspnea of preterm infants and since then it has been widely used in pediatrics, particularly in pediatrics with respiratory failure due to bronchiolitis. Especially, HFNC has been used frequently during Covid-19 pandemic.
Currently, HFNC is considered as a respiratory support device in the indications for severe patients: respiratory failure due to hypoxia, exacerbation of chronic obstructive pulmonary disease, after extubation, before airway procedure, sleep apnea, immunocompromised patients. Numerous published reports have shown that HFNC reduces respiratory rate and work, reducing the need to escalate respiratory support in patients with various underlying medical conditions. HFNC includes air and oxygen mixer, humidifier, and gas conductors with the function of warming the gas coming via a soft material nose canula.
The HFNC provides a flow of warmed and humidified air at up to 60 L/min, resulting in several physiological benefits: reduction of anatomical dead space, generation of PEEP (positive end-expiratory pressure), correction of the fraction of oxygen in inhaled air (FiO2) is independent of air flow rate, providing a flow of air that is warm and humid enough to not injure the airways.
KEYWORDS: HFNC; Covid-19; Respiratory Faillure; High flow; Hypoxia.
RÉSUMÉ
Le HFNC (haut débit à canule nasal) a été introduit pour la première fois à la pratique clinique au début de l’année 2000 comme le système non-invasive pour le traitement des nouveau-né préterm; et après il est utilisé très souvent dans la pédiatrie, en particulier chez les enfants ayant l’insuffisance respiratoire à cause de la bronchiolite. Particulièrement, le HFNC a été utilisé très souvent pendant la pandémie du Covid-19.
Malgré il n y a pas d’essaie clinique avec une suffisamment large échantillon, le traitement avec le HFNC a eu une attention des réanimateurs grâce à son efficacité. Actuellement, le HFNC a été considéré comme l’appareil à support ventilatoire dans les indications pour les patients critiques: l’insuffisance respiratoire à cause de l’hypoxémie, l‘exacerbation de la bronchopneumopathie chronique obstructive, après l’extubation, avant l’intervention des voies aériennes, les apnées du sommeil, et chez les patients avec le déficit immunitaire.
Plusieurs études ont démontrées que le HFNC diminue la fréquence respiratoire et la charge du travail, la besoin de monter le support respiratoire chez les patients avec des différentes conditions de maladies. Le HFNC compris l’air et le mixer d’oxygène, l’humidificateur, le conducteur de l’air avec la fonction de réchauffement via au canular flexible et mou. Le HFNC fournie de l’air tiède et humide avec 60 L/min, ayant plusieurs bénéfices physiologiques: réduire l’espace morte anatomique, créer la PEEP (pression positive à la fin expiratoire), corriger la fraction d’oxygène de l’air inhalé (FiO2) de façon indépendante avec débit, produire de l’air tiède et humide pour éviter des lésions des voies aeriennes.
MOTS CLÉS: HFNC; Covid-19; Insuffisance respiratoire; Haut débit; Hypoxie.
INTRODUCTION
Increasing airway pressure and increasing FiO2 improve blood oxygenation by different mechanisms and may be optimal at higher flow rates. Most of the effects of high flow nasal canular (HFNC) are related to the elimination of lethal factors such as increased CO2 productions, decreased respiratory regulation, and increased respiratory rate or respiratory effort. This benefit can not be obtained by lower flows. All of these physiological effects may indicate the comfort in patients with respiratory failure and the efficacy of HFNC [1].
HFNC includes air and oxygen mixer, humidifier, and gas conductors with the function of warming the gas coming via a soft material nose canula. The HFNC provides a flow of warmed and humidified air at up to 60 L/min, resulting in several physiological benefits: reduction of anatomical dead space, generation of PEEP (positive end-expiratory pressure), correction of the fraction of oxygen in inhaled air (FiO2) is independent of air flow rate, providing a flow of air that is warm and humid enough to not injure the airways.
HFNC was first introduced into clinical practice in the early 2000s as a non-invasive system for the management of dyspnea of preterm infants and since then it has been widely used in pediatrics, particularly in pediatrics with respiratory failure due to bronchiolitis [2].
Although to date there are no clinical trials with a sufficiently large sample size, HFNC therapy has nevertheless attracted the attention of resuscitators due to its clear efficacy [3].
Currently, HFNC is considered as a respiratory support device in the indications for severe patients: respiratory failure due to hypoxia, exacerbation of chronic obstructive pulmonary disease (COPD), after extubation, before airway procedure, sleep apnea, or immunocompromised patients.
Numerous published reports have shown that HFNC reduces respiratory rate and work, reducing the need to escalate respiratory support in patients with various underlying medical conditions or acute respiratory distress syndrome (ARDS) in Covid-19 patients [4-7].
It is because of HFNC effectiveness, the use of this new device and technique has spread beyond the intensive care units (ICUs) to the respiratory disease departments. It is widely and increasingly used as an alternative to NIV (non invasive ventilation) when it fails from a variety of causes.
Finally, over the past few years, technical developments have made HFNC devices simpler and more user-friendly, suitable for both hospital healthcare and home use, giving clinicians more therapeutic options.
ADVANTAGES OF HFNC
Adjustable FiO2
Treatment of HFNC exerts benefits on the respiratory system by a number of different mechanisms. However, it is not clear which benefit is most important, depending on the cause of the individual patient's respiratory failure. Several mechanisms have been explored, but others remain unclear. In daily clinical practice, HFNC quickly became widely adopted, while physiological studies are still ongoing. The air from this system (HFNC) mixes air and oxygen which can produce a total flow rate of up to 60 L/min which is warmed and humidified by an active humidifier then passed through a heating circuit. High flow of fully warmed and humidified gas provides physiological effects (Table 1):
1. High flow helps to eliminate CO2 in the anatomical dead space.
2. Overcoming the resistance of expiratory flow and create positive pressure in the nasopharynx. Although airflow is delivered through an open system, the high flow rate and although this pressure is relatively low compared with other closed systems, is sufficient to increase lung volume or increase the recruitment capacity of collapsed alveoli.
3. The difference between the patient inspiratory air flow and the delivered air flow is small and the supplied FiO2 is relatively constant.
4. Reducing the work of breathing, improve the patient's chest-abdominal synchronization.
5. The gas is warmed at 37°C and moist, the mucosal functions are well maintained and causes little discomfort to the patient.
DISADVANTAGES OF HFNC
Delay invasive ventilation-optimal intubation time
In a retrospective study to evaluate the potential for HFNC use to delay intubation and potentially harmful side effects, authors Kang et al. noted the use of HFNC in more than 48 hours prior to intubation may be associated with increased ICU mortality, reduced weaning rates, extubation, and ventilator-free days [8]. In another study, in patients with COPD exacerbations, 20% of patients failed to use NIV despite a good 48-hour response to NIV, and these patients had a very poor in-hospital prognosis, when compared with patients who were intubated on mechanical ventilation early on [9].
These studies have raised the alarm about the risk that inappropriate use of noninvasive modalities, including HFNC and NIV, can lead to adverse effects. Furthermore, because HFNC is more comfortable for the patient than NIV and can be tolerated for a longer period of time, it is more likely that the wrong timing of intubation is more likely to occur. Because delayed intubation can worsen prognosis in HFNC-treated patients, predictors of failure are of particular interest. One study showed decreased respiratory rate, reduced oxygenation and thoracic-abdominal asynchrony are predictors of HFNC failure [10]. Or more recently, in 2019, Roca's study showed that ROX index has the ability to predict the outcome of HFNC therapy in pneumonia patients with acute respiratory failure [11]. HFNC is widely applied to patients with various underlying diseases; response to HFNC will therefore vary depending on the underlying disease.
Unlike standard oxygen therapy, HFNC is actually a very powerful noninvasive respiratory support therapy, equivalent to NIV. Therefore, when HFNC is no longer able to support the patient, meaning that the patient has severe respiratory failure, it should be considered for early mechanical ventilation intubation. Currently, there are no studies large enough to define criteria to accurately choose the timing of intubation when patients are receiving HFNC ventilation. However, some authors recommend that we closely monitor patients breathing HFNC during the first hour: in patients who respond well to HFNC, often respiratory and hemodynamic parameters are improved within 1 hour. HFNC is an open ventilation system but it can still increase end-expiratory pressure. Oropharyngeal pressure is affected by the opening or closing of the mouth, the air supply, and the size of the nasal canula.
Pneumothorax
Previous authors such as Hedge and Prodhan reported 3 cases of severe pneumothorax associated with HFNC therapy: pneumothorax in a twomonth -old boy with respiratory syncytial virus bronchitis with 8 L/min; pneumomediastinum in a 16-year-old
male patient with cerebral palsy receiving 20 L/min; and pneumothorax (right) in a 22-month-old boy with a subdural hematoma receiving 6 L/min [12]. While the ventilation system in the pediatric patient has been incorporated with a safety valve to avoid high pressure, this system for adults has no pressure relief valve nor pressure monitoring. Therefore, such complications can also occur in adult patients.
However, the knowledge about using HFNC in other diseases such as lung fibrosis with acute respiratory faillure due to systemic sclerosis, severe pneumonia, exacerbation of COPD and the use of non-invasive biomarker of exhaled nitric oxide has not been well studied [13-16]. Especially, the human resources needing for patient care is also a crucial factor to assure the succes of HFNC treatment [17].
INDICATIONS AND CONTRAINDICATIONS
Indications
Patients with severe COVID-19 pneumonia with:
Contraindications
Contraindications for HFNC mainly focus on airway protection and upper airway abnormalities. In addition, several medical conditions warrant the careful indication of HFNC.
Relative contraindications
Contraindications to HFNC in infants and young children
Usual caution with HFNC in infants and young children
Absolute contraindication
IMPLEMENTATION PROCESS
Prepare
Medical staff
A respiratory specialist, CPR or a doctor trained in HFNC is required, usually these people will be the top decision-makers.
Ensure 1 nurse takes care of 2 – 3 critically ill patients (1st level care). It is necessary to have enough to ensure safety when applying the procedure, to avoid a lack of manpower leading to failure to detect and promptly handle situations of complications or failures requiring intubation.
Materials
HFNC machine: comprising compressed air and oxygen flow mixing system with safety valve with maximum air flow (Flow) of 140 L/min and oxygen pressure (FiO2) from 21 to 100%, humidification and heating system. volume of mixed gas. Care should be taken to prepare the humidification system carefully, as it can obstruct the airways due to sputum congestion (Figure 1).
Consumables: specialized nasal cannula: usually used once, but in practice can be sterilized to be reused 1-2 more times.
Sterile plastic airway kit (breathing line).
Oxygen system (wall oxygen or oxygen tank with pressure reducing valve): should prepare a central oxygen system to ensure the flow and pressure to achieve the maximum FiO2 and at the same time avoid oxygen pressure drop at other sites lead to local hypoxia.
Compressed air system (or air compressor): there should be a central compressed air system to ensure the pressure source.
- Suction system (or portable vacuum cleaner).
- Continuous monitoring machine: Electrocardiogram, blood pressure, and SpO2.
Each patient should be equipped with a monitor for continuous monitoring. In the condition that there are not enough facilities, priority should be given to new patients indicated within the first 1-2 hours.
- Blood gas testing machine.
- Bedside X-ray machine.
- Ambu ball with mask, oxygen breathing kit (oxymeter, oxygen humidifier, oxygen tube, oxygen goggles, and oxygen mask)
- Emergency pleural dialysis, low-pressure air suction system, first aid kit to stop circulation.
Prepare the HFNC system
+ Plug in the power of the HFNC.
+ Install the air line into the HFNC system.
+ Insert the drip infusion air lines.
+ Connect the canulla to the airway.
+ Adjust the air flow accordingly, usually starting at 40 L/min, can increase ubottle into the humidifier, plug in the heating and humidifying system.
+ Connect oxygen and compressed p to 60 L/ minute, depending on the comfort level of the patient. Always check to make sure there is a line.
+ Adjust FiO2.
Patients
- Explain to the patient and his/her family/legal representative about the need and risks of HFNC. The patient/patient's representative signs a commitment to perform the technique (Figure 2).
- Do a blood gas test.
- Measure blood pressure, take pulse, breathing rate, and SpO2.
- Set the monitor to monitor continuously.
Medical records
- Complete recording of parameters to be monitored.
- Check the test results.
Steps to take
- Check the HFNC system.
- Check the patient.
- Perform.
- Adjust HFNC parameters.
- Assess the patient.
Check the HFNC system
Check once more about the power source, oxygen source, compressed air to ensure good operation.
Set initial parameters
- 100% FiO2.
- Flow: 30-40 L/min.
Check the patient's condition to see if the instructions are correct or not.
Check up on the patient
Explain and guide the patient to know the benefits of HFNC breathing and how to breathe correctly to
achieve the highest efficiency. Encourage the patient to cooperate with the correct technique.
Perform
- Connect the cannula to the patient and fix the cannula: Make sure it is enough to not fall, should not be fixed too tightly to make the patient uncomfortable.
- After the connection, the doctor must continue to observe and evaluate the effectiveness of the technique. Ensure patient comfort and clinical improvement.
- Monitor SpO2, pulse, blood pressure, and breathing rate.
- Do a blood gas test after 60 minutes of breathing with HFNC.
Objectives to be achieved
+ SpO2 95 - 96% (patients with COPD only need to reach > 92%), or PaO2 >60 mmHg.
+ PaCO2 is normal or acceptable pH (when ventilation is acceptable): increased CO2 in patients with ARDS, bronchial asthma, or COPD.
+ Breathing rate ≤30 times/minute.
For severe COVID-19 patients during the pandemic: a target respiratory rate ≤30 breaths/min and SpO2 > 92% can be accepted with any FiO2 within the first 2-6 hours: To reduce overload for invasive mechanical ventilation.
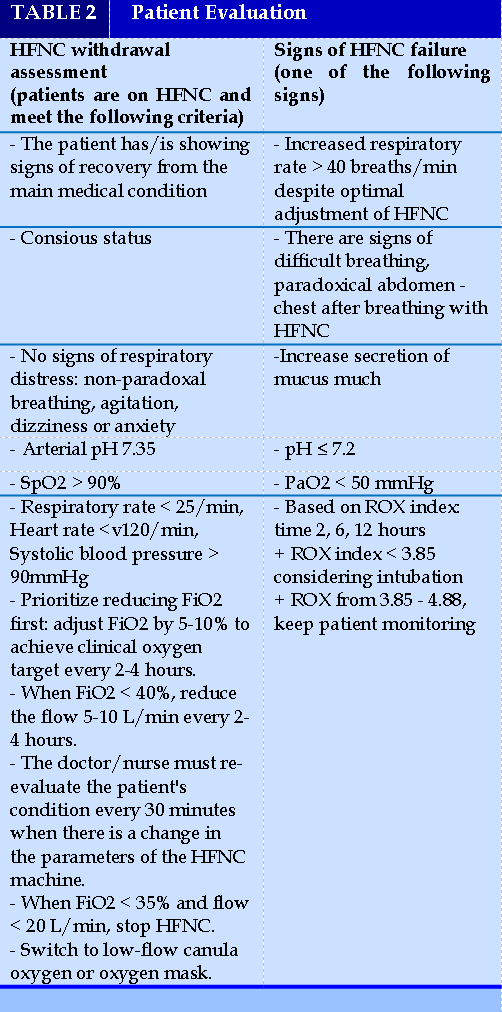
Adjust the HFNC parameter (Table 2)
- The doctor should stand next to the patient at least for the first 15 - 30 minutes to assess and make the earliest and most timely adjustment.
- Adjust FiO2 up or down by 5-10% every 10-15 minutes to achieve clinical oxygen target.
- Adjust the flow rate up or down by 5-10 L/minute every 10-15 minutes, up to 60 L/minute depending on the needs and response of the patient.
+ If breathing rate increases: priority to increase flow first until optimal flow is reached. In case it is accompanied by a decrease in SpO2, the FiO2 will be increased at the same time to reach the target SpO2
+ If the target oxygen is not reached (SpO2 < 92%): prioritizing increase FiO2 to reach the target SpO2. Then further adjust the flow to achieve the target SpO2 with the lowest FiO2 while achieving the best flow that is comfortable for the patient.
- Evaluation of HFNC withdrawal
- HFNC failure indicators
FOLLOW-UP OF PATIENTS WITH HFNC
- Make sure the machine is always in good working condition:
- Monitoring the patient's condition:
+ The patient tolerates well:
Consciousness improves or does not worsen, SpO2 is stable or increased, pulmonary ventilation is good, pulse and blood pressure are stable, breathing rate does not increase more than 20% compared to initial parameters, no signs of breathing muscle contractions.
+ Patients with intolerance:
- Arterial blood gas test:
- Chest X-ray:
Take 1-2 times/3—5day; doing emergency scan when necessary.
- Assess and monitor the patient's response to oxygen therapy by clinical and blood gas testing. Immediately after giving the patient oxygen for about 15 minutes, we conduct blood gas and record clinical changes.
- Facial color: red pink can be an excess of oxygen, purple can be a lack of oxygen.
- Monitor respiratory rate, breathing pattern, continuous SpO2, heart rate, HFNC wiring, patient response per hour, and arterial blood gas results.
- Monitor signs of dyspnea and consequences of breathing on patients; hemodynamic instability; SpO2 < 90% with FiO2 > 60%; reduced level of consciousness Glasgow < 10 points; gastric retention, paralytic ileus, persistent vomiting, risk of aspiration pneumonia.
- Ensure airway ventilation: instruct the patient to cough, sputum or suction for the patient.
- Pay attention to ensure that the humidifying temperature is maintained at 36-37 degrees; the humidifier is always fully supplied with water.
- Depending on the cause, the tracking distance may vary. If the patient has COPD, due to the risk of depression of the respiratory center, more close monitoring is required.
+ If after 30-60 minutes of oxygen, clinical improvement, SpO2 > 90%, maintain oxygen and treat the cause.
+ But if there is no clinical improvement, SpO2 < 90%, then do blood gas to adjust according to the blood gas result.
+ If the patient's condition worsens in combination with blood CO2 levels, non-invasive mechanical ventilation is indicated (Figure 3).
If consciousness worsens, invasive ventilation should be intubated.
Patients with severe COVID-19 pneumonia should lie on their stomach to improve blood oxygenation. In this case, monitoring the patient is difficult, requires closer monitoring, requires more manpower and better training (Figure 4).
- Pay attention to the patient's nutrition.
+ Provide enough energy, nutrients, water to improve health and immunity.
+ Oral nutrition for patients who can still eat with regular food and supplement at least one snack with milk/standard nutritional soup/high energy, high protein.
+ Early enteral nutrition (within 48 hours immediately after hemodynamic control) in critically ill patients to maintain gastrointestinal and immune function.
+ Early parenteral nutrition when enteral nutrition has contraindications or when energy and protein requirements are not met.
+ Supplement vitamins and microelements with the minimum basic dose to ensure cell metabolism and immunity.
- Prevention of hospital-acquired infections:
+ Hand hygiene (hand washing, alcohol sanitizer, gloves).
+ Oral hygiene (moisturizing, brushing teeth, disinfecting oral cavity with chlorhexidine).
+ Management of breathing lines (draining condensate, do not change periodically).
- Prophylaxis of gastric and duodenal ulcers (Sucralfate, proton pump inhibitors - PPIs, H2 receptor antagonists).
- Prophylaxis of deep vein thrombosis.
COMPLICATIONS AND MANAGEMENT
- Consciousness:
It is necessary to monitor whether the patient's consciousness is awake (coma: causes of respiratory acidosis, worsening respiratory failure...), if the patient is comatose, treat intubation and mechanical ventilation (Table 3).
- Dry, burning nasal mucosa causing pain:
Immediately check the heating and humidification system for water.
If it is, check if the set temperature is high.
Adjust Flow to ensure target oxygen and patient comfort.
- Hypotension:
When the mean blood pressure is below 65 mmHg
Monitor blood pressure.
Treatment of hypotension: perfusion, use vasopressors if necessary. Treat the cause of low blood pressure (septic shock, volume depletion, etc.)
Pressure injuries: these are dangerous, potentially life-threatening complications. Therefore, it should be detected early and treated promptly.
- Pneumothorax under the skin of the neck and chest: The patient's symptoms may be normal, there is no respiratory failure, but there is swelling in the neck and face. Feel the squishy mark under the skin.
Treatment: Adjust Flow to reduce pressure, in case of many causes causing respiratory failure, then puncture with needle 18 to remove air; or endotracheal intubation.
- Pneumomediastinum: showing signs of subcutaneous pneumothorax, possibly respiratory failure, cardiac tamponade causing hypotension.
Management: aspiration mediastinal air right in the intercostal space 2—3th; close to the left breast edge, puncture perpendicular to the chest wall.
- Pneumothorax: manifestations of dyspnea, irritability, resistance to machine, SpO2 drop, subcutaneous pneumothorax, pulmonary examination showing signs of pneumothorax.
Treatment: placing emergency pleural drainage.
REFERENCE
1. Use of nasal high flow oxygen during acute respiratory failure. Intensive Care Medicine volume 46, pages2238-2247; 2020.
2. Parke R. L., Bloch A., McGuinness S. P. Effect of Very-High-Flow Nasal Therapy on Airway Pressure and End-Expiratory Lung Impedance in Healthy Volunteers. Respir Care, 2015;60 (10), 1397-403.
3. Kang H., Zhao Z., Tong Z. Effect of High-Flow Nasal Cannula Oxygen Therapy in Immunocompromised Subjects With Acute Respiratory Failure. Respir Care, 2020; 65 (3), 369-376.
4. Duong-Quy S, Huynh-Truong-Anh D, Q Tran-Xuan et al. Bradycardia unresponded to atropin testing was successfully treated with therapeutic plasma exchange in a patient with severe COVID-19 complicated by Guillain-Barré syndrome: A case report. Frontiers in Cardiovascular Medicine, 2022.
5. Duong-Quy S, D Huynh-Truong-Anh, T Nguyen-Thi-Kim, et al. The use of therapeutic plasma exchange in the treatment of a pregnant woman with COVID-19 induced acute respiratory distress syndrome Pulmonary Therapy 2022; 8 (2), 233-240.
6. Duong-Quy S, Huynh-Truong-Anh D, Nguyen-Thi-Kim T, Nguyen-Quang T, Nguyen-Chi T, Tran-Xuan Q, Nguyen-Nhu V, Ngo C, Craig T. Guillain-Barré Syndrome in Patient With SARS-CoV-2 PCR Positivity Treated Successfully With Therapeutic Exchange Plasma: A First Case Report From Vietnam. Frontiers in Neurology. 2022;13.
7. Duong-Quy S, D Huynh-Truong-Anh, N Le-Thi-Hong, T Le-Van, et al. Acute respiratory distress syndrome associated with multisystem Inflammatory syndrome in a child with Covid-19 and diabetic ketoacidosis: a case report Pulmonary Therapy 2022;8 (3), 333-342.
8. Epstein A. S., Hartridge-Lambert S. K., Ramaker J. S., et al. (2011), "Humidified high-flow nasal oxygen utilization in patients with cancer at Memorial Sloan-Kettering Cancer Center", J Palliat Med, 14 (7), 835-9.
9. Itagaki T., Okuda N., Tsunano Y., et al. Effect of high-flow nasal cannula on thoraco-abdominal synchrony in adult critically ill patients", Respir Care, 2014;59 (1), 70-4.
10. Parke R., McGuinness S., Dixon R., et al. Open-label, phase II study of routine high-flow nasal oxygen therapy in cardiac surgical patients. Br J Anaesth 2013; 111 (6), 925-31.
11. Moretti M., Cilione C., Tampieri A., et al. Incidence and causes of non-invasive mechanical ventilation failure after initial success. Thorax 2000; 55 (10), 819-25.
12. Conti G., Antonelli M., Navalesi P., et al. Noninvasive vs. conventional mechanical ventilation in patients with chronic obstructive pulmonary disease after failure of medical treatment in the ward: a randomized trial. Intensive Care Med, 2022; 28 (12), 1701-7.
13. Hua-Huy T, Le-Dong NN, Duong-Quy S, Bei Y, Rivière S, Tiev KP, Nicco C, Chéreau C, Batteux F, Dinh-Xuan AT. Increased exhaled nitric oxide precedes lung fibrosis in two murine models of systemic sclerosis. Journal of Breath Research. 2015 Jun 16;9(3):036007-.
14. Nguyen Thi Dieu T, A Pham Nhat, TJ Craig, et al. Clinical characteristics and cytokine changes in children with pneumonia requiring mechanical ventilation. Journal of International Medical Research 2017; 45 (6), 1805-1817.
15. Duong-Quy S, H Tran Van, A Vo Thi Kim, Q Pham Huy, TJ Craig. Clinical and functional characteristics of subjects with asthma, COPD, and asthma-COPD overlap: a multicentre study in Vietnam. Canadian Respiratory Journal 2018.
16. Duong-Quy S, Nguyen-Thi-Dieu T, Tran-Quang K, Tang-Thi-Thao T, Nguyen-Van T, Vo-Pham-Minh T, Vu-Tran-Thien Q, Bui-Diem K, Nguyen-Nhu V, Hoang-Thi L, Craig T. Study of nasal fractional exhaled nitric oxide (feno) in children with allergic rhinitis. Sinusitis. 2021 Oct 8;5(2):123-31.
17. Duong-Quy S, Tran-Duc S, Hoang-Chau-Bao D, Bui-Diem K, Vu-Tran-Thien Q, Nguyen-Nhu V. Tiredness, depression, and sleep disorders in frontline healthcare workers during COVID-19 pandemic in Vietnam: A field hospital study. Frontiers in psychiatry. 2022 Oct 17;13:984658.
FIGURE - TABLES

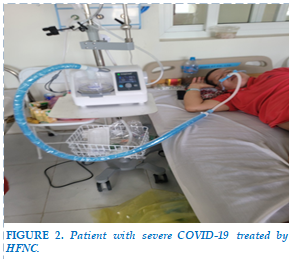
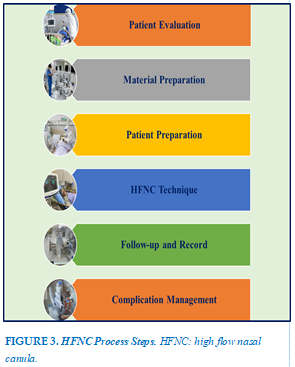
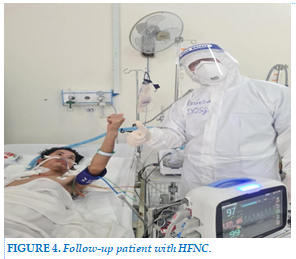
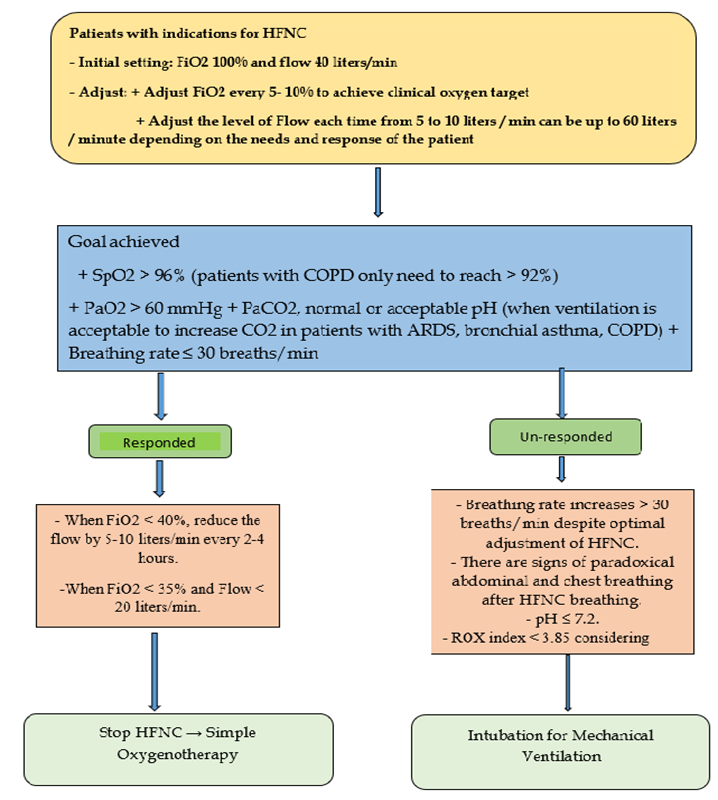
FIGURE 5. HFNC Flow-Chart Process.
HFNC: high flow nasal canula.


REFERENCE
1. Use of nasal high flow oxygen during acute respiratory failure. Intensive Care Medicine volume 46, pages2238-2247; 2020.
2. Parke R. L., Bloch A., McGuinness S. P. Effect of Very-High-Flow Nasal Therapy on Airway Pressure and End-Expiratory Lung Impedance in Healthy Volunteers. Respir Care, 2015;60 (10), 1397-403.
3. Kang H., Zhao Z., Tong Z. Effect of High-Flow Nasal Cannula Oxygen Therapy in Immunocompromised Subjects With Acute Respiratory Failure. Respir Care, 2020; 65 (3), 369-376.
4. Duong-Quy S, Huynh-Truong-Anh D, Q Tran-Xuan et al. Bradycardia unresponded to atropin testing was successfully treated with therapeutic plasma exchange in a patient with severe COVID-19 complicated by Guillain-Barré syndrome: A case report. Frontiers in Cardiovascular Medicine, 2022.
5. Duong-Quy S, D Huynh-Truong-Anh, T Nguyen-Thi-Kim, et al. The use of therapeutic plasma exchange in the treatment of a pregnant woman with COVID-19 induced acute respiratory distress syndrome Pulmonary Therapy 2022; 8 (2), 233-240.
6. Duong-Quy S, Huynh-Truong-Anh D, Nguyen-Thi-Kim T, Nguyen-Quang T, Nguyen-Chi T, Tran-Xuan Q, Nguyen-Nhu V, Ngo C, Craig T. Guillain-Barré Syndrome in Patient With SARS-CoV-2 PCR Positivity Treated Successfully With Therapeutic Exchange Plasma: A First Case Report From Vietnam. Frontiers in Neurology. 2022;13.
7. Duong-Quy S, D Huynh-Truong-Anh, N Le-Thi-Hong, T Le-Van, et al. Acute respiratory distress syndrome associated with multisystem Inflammatory syndrome in a child with Covid-19 and diabetic ketoacidosis: a case report Pulmonary Therapy 2022;8 (3), 333-342.
8. Epstein A. S., Hartridge-Lambert S. K., Ramaker J. S., et al. (2011), "Humidified high-flow nasal oxygen utilization in patients with cancer at Memorial Sloan-Kettering Cancer Center", J Palliat Med, 14 (7), 835-9.
9. Itagaki T., Okuda N., Tsunano Y., et al. Effect of high-flow nasal cannula on thoraco-abdominal synchrony in adult critically ill patients", Respir Care, 2014;59 (1), 70-4.
10. Parke R., McGuinness S., Dixon R., et al. Open-label, phase II study of routine high-flow nasal oxygen therapy in cardiac surgical patients. Br J Anaesth 2013; 111 (6), 925-31.
11. Moretti M., Cilione C., Tampieri A., et al. Incidence and causes of non-invasive mechanical ventilation failure after initial success. Thorax 2000; 55 (10), 819-25.
12. Conti G., Antonelli M., Navalesi P., et al. Noninvasive vs. conventional mechanical ventilation in patients with chronic obstructive pulmonary disease after failure of medical treatment in the ward: a randomized trial. Intensive Care Med, 2022; 28 (12), 1701-7.
13. Hua-Huy T, Le-Dong NN, Duong-Quy S, Bei Y, Rivière S, Tiev KP, Nicco C, Chéreau C, Batteux F, Dinh-Xuan AT. Increased exhaled nitric oxide precedes lung fibrosis in two murine models of systemic sclerosis. Journal of Breath Research. 2015 Jun 16;9(3):036007-.
14. Nguyen Thi Dieu T, A Pham Nhat, TJ Craig, et al. Clinical characteristics and cytokine changes in children with pneumonia requiring mechanical ventilation. Journal of International Medical Research 2017; 45 (6), 1805-1817.
15. Duong-Quy S, H Tran Van, A Vo Thi Kim, Q Pham Huy, TJ Craig. Clinical and functional characteristics of subjects with asthma, COPD, and asthma-COPD overlap: a multicentre study in Vietnam. Canadian Respiratory Journal 2018.
16. Duong-Quy S, Nguyen-Thi-Dieu T, Tran-Quang K, Tang-Thi-Thao T, Nguyen-Van T, Vo-Pham-Minh T, Vu-Tran-Thien Q, Bui-Diem K, Nguyen-Nhu V, Hoang-Thi L, Craig T. Study of nasal fractional exhaled nitric oxide (feno) in children with allergic rhinitis. Sinusitis. 2021 Oct 8;5(2):123-31.
17. Duong-Quy S, Tran-Duc S, Hoang-Chau-Bao D, Bui-Diem K, Vu-Tran-Thien Q, Nguyen-Nhu V. Tiredness, depression, and sleep disorders in frontline healthcare workers during COVID-19 pandemic in Vietnam: A field hospital study. Frontiers in psychiatry. 2022 Oct 17;13:984658.
ARTICLE INFO DOI: 10.12699/jfvpulm.14.41.2023.13
Conflict of Interest
Non
Date of manuscript receiving
15/01/2023
Date of publication after correction
15/06/2023
Article citation
Duong-Quy S, Huynh Truong Anh D, Nguyen Thi Kim T, Nguyen Quang T, Tran Ngoc Anh T, Nguyen Van Hoai N, Do Thi Thu M, Nguyen-Van T, Tang-Thi-Thao T, Nguyen-Tuan A, Trinh Du T, Tran Thai T, Nguyen Van T. Clinical process in indications and application of HFNC therapy in patients with severe Covid-19. J Func Vent Pulm 2023;41(14):13-21