
 English
English
 French
French
Hospital prevalence and etiological profile of bronchiec-tasis in Burkina Faso
Prévalence hospitalière et profil étiologique des bronchectasies au Burkina Faso
AR. Ouedraogo1,2, D. Zida2, G. Bougma2, A. Sourabie3, A. Ouedraogo2, G. Ouedraogo2, E Birba3, K Boncoungou2, G Badoum2, M Ouedraogo2
1: Pneumology Department, TENGANDOGO University Hospital Center. Ouagadougou - Burkina Faso
2: Respirology Department, Yalgado OUEDRAOGO University Hospital Center. Ouagadougou - Burkina Faso
3: Pneumology Department, Souro SANOU University Hospital Center. Bobo Dioulasso - Burkina
Corresponding author:
Abdoul Risgou OUEDRAOGO. Center Hospitalier Universitaire de Tengandogo, Service de Pneumologie, 18 BP 133 Ouagadougou, Burkina Faso. E-mail: oarisgou@yahoo.fr
ABSTRACT
Introduction. Once considered an orphan disease, bronchiectasis represents a real public health problem because of its disabling nature. Its prognosis depends on etiology and early treatment. The data in the literature come mainly from Western countries. The aim of this study is to determine the prevalence and etiological profile of bronchiectasis at the Yalgado Ouédraogo University Hospital Center (CHU-YO) in Burkina Faso.
Method. This was a cross-sectional, retrospective study that took place from January 1, 2016 to December 31, 2018 and involved the records of patients with bronchiectasis seen in the department of Pneumology in of CHU-YO.
Results. Seventy-two cases of bronchiectasis were included out of 2,051 hospitalized patients, a prevalence of 3.51%. The mean age was 58.35 ± 15.39 years and the sex ratio was 1.6. Diffuse and cystic bronchiectasis accounted for 87.69% and 75.38% of the workforce, respectively. The etiologies found were sequelae of pulmonary tuberculosis (26.15%), asthma (23.08%), recurrent lung disease (13.84%), COPD (7.69%), connective tissue disease (1.54%) and allergic bronchopulmonary aspergillosis (1.54%). In 16.92% of cases an etiology was not found.
Conclusion. the etiologies of bronchiectasis were dominated by the sequelae of tuberculosis. However, the inaccessibility of some diagnostic tools makes it difficult to systematically search for other etiologies mentioned in the literature.
KEYWORDS: Bronchiectasis; etiologies; tuberculosis; Burkina Faso; prevalence; bronchiectasis; etiology.
RÉSUMÉ
Introduction. Autrefois considérée comme une maladie orpheline, la bronchectasie représente un véritable problème de santé publique du fait de son caractère invalidant. Son pronostic dépend de l'étiologie et du traitement précoce. Les données de la littérature proviennent principalement des pays occidentaux. Le but de cette étude est de déterminer la prévalence et le profil étiologique des bronchectasies au Centre Hospitalier Universitaire Yalgado Ouédraogo (CHU-YO) au Burkina Faso.
Méthode. Il s'agit d'une étude transversale rétrospective qui s'est déroulée du 1er janvier 2016 au 31 décembre 2018 et qui a porté sur les dossiers de patients atteints de bronchectasie vus au service de pneumologie du CHU-YO.
Résultats. Soixante-douze cas de bronchectasies ont été inclus sur 2 051 patients hospitalisés, soit une prévalence de 3,51 %. L'âge moyen était de 58,35 ± 15,39 ans et le sexe ratio était de 1,6. Les bronchectasies diffuses et kystiques représentaient respectivement 87,69 % et 75,38 % de l'effectif. Les étiologies retrouvées étaient des séquelles de tuberculose pulmonaire (26,15%), d'asthme (23,08%), de pneumopathie récidivante (13,84%), de BPCO (7,69%), de connectivite (1,54%) et d'aspergillose broncho-pulmonaire allergique (1,54%). Dans 16,92% des cas une étiologie n'a pas été retrouvée.
Conclusion. les étiologies des bronchectasies étaient dominées par les séquelles de la tuberculose. Cependant, l'inaccessibilité de certains outils diagnostiques rend difficile la recherche systématique d'autres étiologies évoquées dans la littérature.
MOTS CLÉS: bronchectasie ; étiologies; tuberculose; Burkina Faso; prévalence; bronchectasie; étiologie
INTRODUCTION
Bronchiectasis or bronchial dilatation (DDB) is a permanent and irreversible increase in the caliber of the sub-segmental bronchi, whose functions are altered in more or less extensive territories [1]. Bronchiectasis is a chronic disease that can lead to simple but disabling bronchorrhea due to its abundance, hemoptysis and regular superinfections that can sometimes justify clean lobectomies [1]. It represents a real and growing public health problem. In Europe and the United States, the prevalence of the disease has increased by more than 40% in the last ten years [2]. Although substantial progress has been made in the understanding of the epidemiology of bronchiectasis; on a large scale the data come almost exclusively from Western countries [3,4]. Patients with bronchiectasis in these countries are mainly elderly and female, and about 40% of cases are idiopathic [3].
Small cohorts in China and South America suggest that the characteristics of patients in resource-limited countries may be different from those in Europe [5,6]. In resource-limited countries, the etiologies are dominated by tuberculosis. In studies in which thoracic computed tomography (CT) scans were routinely performed in patients treated and declared cured of tuberculosis, moderate or severe bronchiectasis lesions were identified in 40-80% of patients [7,8]. In Asia, tuberculosis is considered an important underlying cause of bronchiectasis [9]. In sub-Saharan Africa there are very few data on bronchiectasis. In Burkina Faso, the characteristics of bronchiectasis are not described. This study therefore reports the hospital prevalence and etiological profile of patients with bronchiectasis in the largest reference hospital in the country.
MATERIALS AND METHODS
This was a cross-sectional, retrospective study that took place over a three-year period from January 1, 2016 to December 31, 2018. This study involved patients hospitalized for bronchiectasis in the pneumology department of the CHU-YO in Ouagadougou, Burkina Faso. All patients of both sexes, 15 years of age and older, who were hospitalized during the study period for bronchiectasis confirmed on chest CT scan were included in this study. Bronchiectasis was confirmed on chest CT on the basis of one of three diagnostic criteria [10]:
A bronchial diameter greater than or equal to one time the diameter of the satellite artery; a bronchial/arterial ratio greater than one (1), achieving the classic kitten ring appearance;
The absence of at least 2 cm of peripheral bronchial taper;
The presence of a visible bronchus at the periphery of
the lung (less than 1 cm from the pleural interface).
Data collection was retrospective; done on an individual survey form completed from the hospitalization register and the medical records of hospitalized patients. The data collected was entered and analyzed using EPI Info software version 7.2.1.0. The raw Pearson Chi2 test or the Fischer exact test were used to compare categorical variables when necessary. Mean values were presented with standard deviation as an index of dispersion. The relationships between the variables were considered statistically associated with a probability threshold of 0.05. The respect of anonymity was ensured by coding the survey forms. The confidentiality of information collected from patients' medical records was respected.
RESULTS
Socio-demographic data
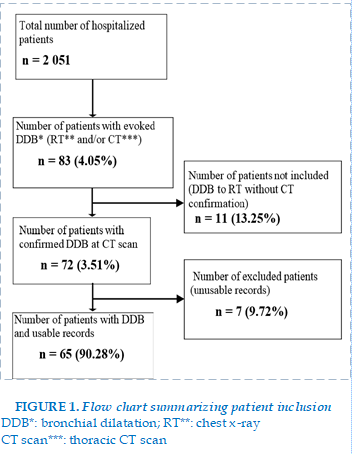
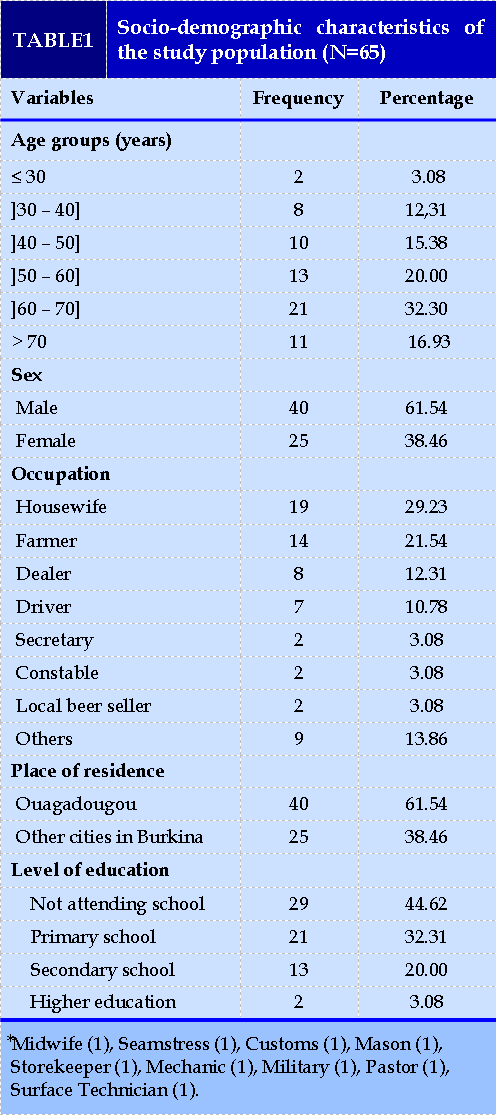
A total of 72 of the 2,051 patients hospitalized during the study period had bronchiectasis, a prevalence of 3.51% (72/2051). Of the 72 patients, 65 (90.28% or 65/72) had a complete clinical record. Figure 1 summarizes the inclusion of patients. The mean (±SD) age was 58.35 (± 15.39) years with extremes of 27 to 95 years. The median age was 60 years, and male patients represented 61.5% of the workforce (40/65). The city of Ouagadougou was the place of residence of 61.54% (40/65) of the patients. Among our patients 29.23% (19/65) were housewives and 44.62% (29/65) had no schooling. Table I presents the socio-demographic characteristics of our population. (FIGURE 1)
Clinical Data
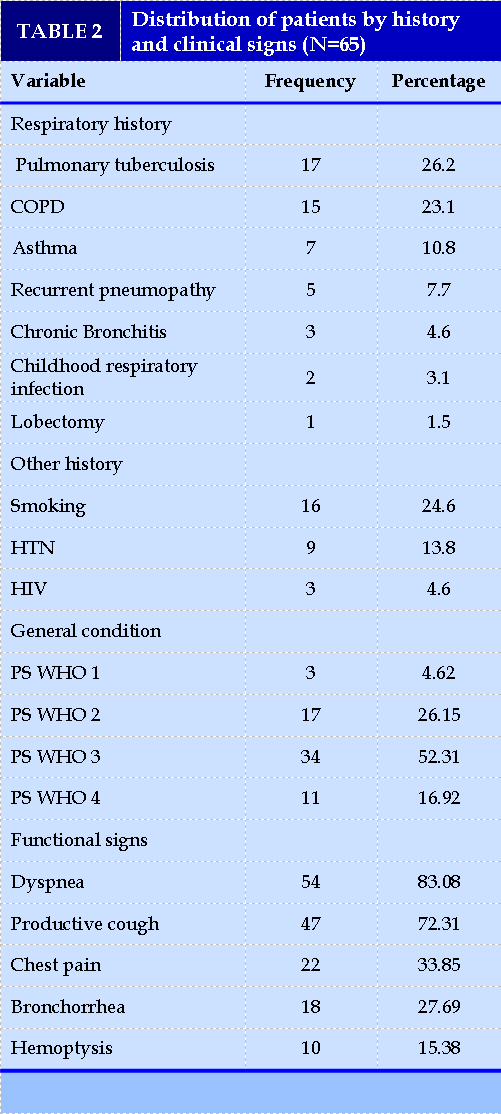
A history of pulmonary tuberculosis and chronic obstructive pulmonary disease (COPD) was found in 26.15% (17/65) and 23.08% (15/65) of patients, respectively.
Smoking was found in 24.61% (16/65) of cases. The finding of bronchiectasis was clinical in 73.85% and incidental in 26.15%. Thirty-four patients or 52.31% (34/65) had a general condition classified as WHO Stage 3 Performance Status. Functional signs were dominated by dyspnea (83.08%;54/65), productive cough (72.31%;47/65) and bronchorrhea (27.69%;18/65). Table II summarizes patients' medical history and clinical signs.
Paraclinical data
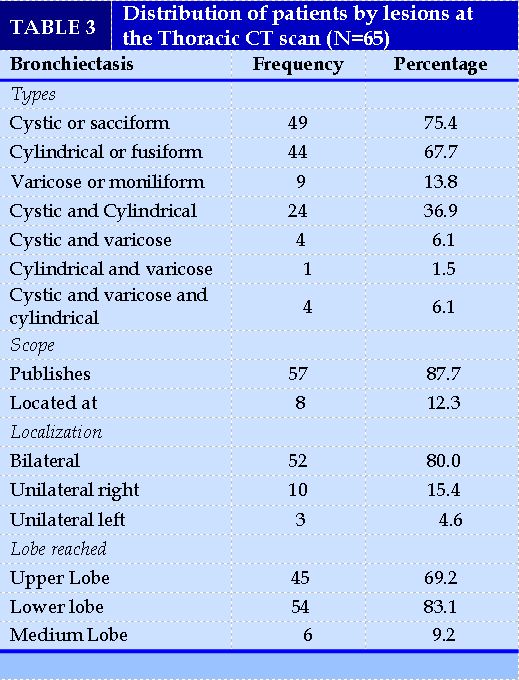
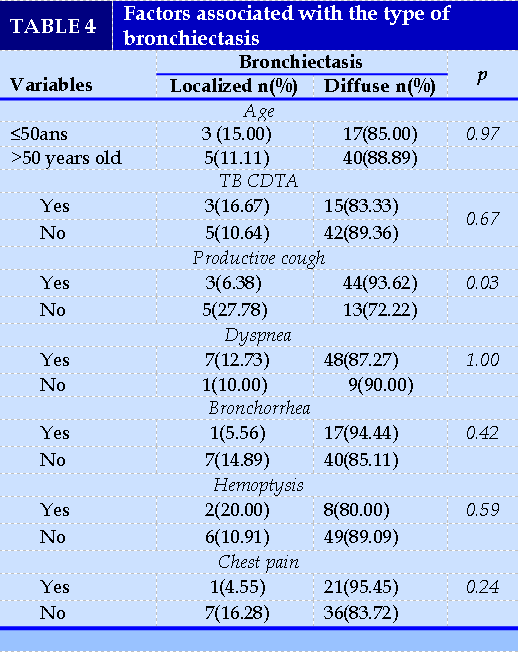
Thoracic CT revealed cystic or sacciform bronchiectasis lesions in 75.38% (49/65) of cases. These lesions were diffuse in 87.69% (57/65) of cases and predominantly in the lower lung lobes in 83.07% (54/65) of cases (Table III). Among the factors associated with the type of bronchiectasis, productive cough was significantly more present in diffuse bronchiectasis (93.62%; 44/47) than in localized bronchiectasis (6.38%; 3/47) with a p-value equal to 0.03 (Table IV).
Bronchial fibroscopy was performed in 21 patients (32.31%; 21/65) and contributed to the diagnosis of allergic bronchopulmonary aspergillosis (ABPA) in one (1) patient. Eleven (11) patients (16.92%; 11/65)
performed spirometry and mixed ventilatory disorder was found in 81.82% (9/11) of cases. Cardiac ultrasonography was performed in 37 patients (56.92%; 37/65). Pulmonary arterial hypertension (PAH) was found in 43.24% (16/37) and chronic pulmonary heart disease in 10.81% (4/37) of cases. (TABLES 3) (TABLES 4)
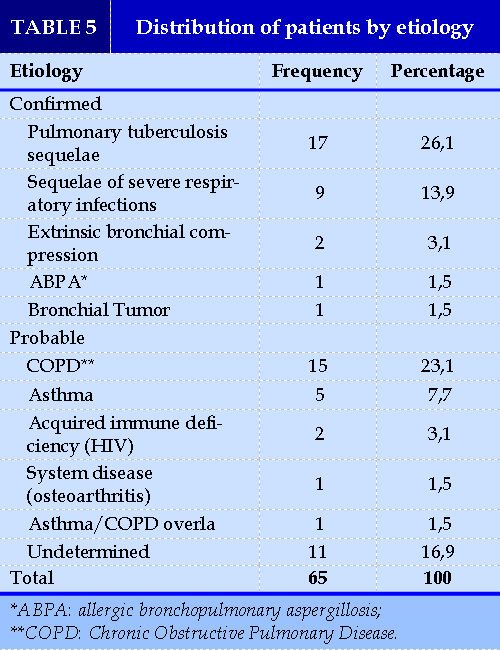
Etiologies
The etiologies found were acquired, dominated by infectious sequelae (40%; 26/65), COPD (23.1%; 15/65) and asthma (7.7%; 5/65). The primary causes were not noted in our study. Extrinsic bronchial compression and allergic bronchopulmonary asper- TABLES 5
gillosis were found in 3.1% (2/65) and 1.5% (1/65) of cases. The causes were undetermined in 16.92% (11/65) of cases (Table V).
DISCUSSION
We aimed to determine the hospital prevalence and etiological profile of bronchiectasis. The prevalence was about 3.51%. This prevalence may be underestimated for a following reasons: first, we did not take include the patients in whom bronchiectasis was suspected but who could not perform chest CT scan for confirmation. Second, because the country, Burkina Faso is in a tuberculosis endemic zone, and according to the literature, the sequelae of pulmonary tuberculosis are DDB. A systematic review of studies that systematically performed chest CT scans in patients cured of tuberculosis reported bronchiectasis in 40-80% of cases [7].
Age is a risk factor for bronchiectasis because it occurs in 75% of patients over 50 years of age [1]. In the present study, the mean age of the patients was 58.35.
Male subjects predominated with 61.5% of patients.
The majority of authors report a female predominance. Gao et al. in a systematic review of the literature in 2016 of 56 studies including 8,608 adults with bronchiectasis reported a female predominance [11 ]. The few studies in which male subjects predominated were in endemic areas [12]. Thus, gender may be related to the etiologies of bronchiectasis. In the West, the causes are dominated by non-tuberculous infectious sequelae, and other pathologies that are more common in females, such as rheumatoid arthritis and Sjögren's syndrome [9]. In countries with limited resources, tuberculosis is thought to dominate the aetiologies; and tuberculosis is more prevalent in men than in women [13].
The onset of bronchiectasis presupposes the conjunction of environmental factors, especially infectious, and a predisposing ground. Thus, a distinction is made between congenital and acquired forms [14]. Congenital forms are mainly the expression of a predisposing ground, namely, cystic fibrosis, a structural anomaly, a humoral or cellular immune deficiency or primary ciliary dyskinesias. Acquired forms may be secondary to a respiratory infection such as tuberculosis, chronic bronchial inflammation, autoimmune pathology or extrarespiratory inflammation, and bronchial compression or obstruction [15].
In our study, etiologies were confirmed in 46.15%. Among the confirmed causes, post infectious causes predominated (40%) including pulmonary tuberculosis (26.1%).
Indeed, various mechanisms are implicated in tuberculosis: bronchial compression by lymphadenopathy, parenchymal destruction or traction by scar tissue [1]. Bronchiectasis is usually encountered in the sequelae of pulmonary tuberculosis [16]. However, it can occur in active forms and it is difficult to determine whether bronchiectasis is related to the current infection or whether it is a sign of a previous infection.
However, the occurrence of bronchiectasis is not only related to pulmonary tuberculosis; there are other factors such as COPD, asthma, and rheumatoid arthritis described in the literature [17,18].
In the present study, other pathologies were found to be the cause of bronchiectasis in 36.9% of cases. Thus, COPD and asthma were represented in 23.1% and 7.7% respectively. COPD would be related to tobacco intoxication (24.6% of our patients). Data from the literature showed a high rate of coexistence of bronchiectasis and COPD, which could be explained by the overlapping pathological mechanisms. Neutrophils and T lymphocytes are the predominant inflammatory cells. Neutrophils release
proteases that cause damage to the lung structure, while T cells cause hypertrophy of the peribronchial lymph nodes and chronic inflammation of the airways, resulting in airway obstruction [19]. In the study by Lonni et al., which included data from nearly 1,300 European patients, COPD appeared to be the third common cause of bronchiectasis (15%) after idiopathic (40%) and post-infectious (20%) forms [20]. Deghdegh et al. in Algeria found the coexistence of COPD and bronchiectasis in 18.75% of patients [21].
In our study, asthma was selected as a probable cause of bronchiectasis. The relationship of asthma to bronchiectasis is described in the literature and its prevalence ranges from 3% to 11% [22]. The influence of asthma on bronchiectasis remains unclear. Bronchiectasis is thought to be found in cases of difficult asthma in older subjects, with a longer duration of the disease. In our study the causes of bronchiectasis were undetermined in 16.92% of cases. Aliberti et al. and Lonni et al. reported 34% and 40% of cases respectively [20,23]. However, in our context, the idiopathic causes can be explained on the one hand by the lack of standardization in the diagnostic approach and on the other hand by the sometimes too rapid linkage to an infectious cause and the too systematic exclusion of congenital forms revealed in adulthood (e.g. cystic fibrosis).
Thus, when faced with bronchiectasis, the etiological diagnosis must be oriented and a certain number of minimal additional tests may be necessary depending on the clinical status. These include sinus CT scan, blood count, plasma protein electrophoresis, rheumatoid factor, antinuclear factors, cryoglobulinemia, immunoglobulin weight determinations, HIV serology, total IgE, aspergillar specific IgE, aspergillar serology; the search for mycobacteria in sputum (± bronchial endoscopy), measurement of nasal nitric oxide, sweat test and the search for the 51 most frequent mutations of the gene coding for the cystic protein fibrosis transmembrane conductance regulator (CFTR) and the search for primitive ciliary dyskinesia or cystic fibrosis [24]. However, these reports are not available in our practice setting or are financially inaccessible to our patients.
The study has limitations related primarily to its retrospective nature. Nevertheless, the data reflect the day-to-day practice of clinicians in our context.
CONCLUSION
This study determined a hospital prevalence of bronchiectasis, even though it appears to be underestimated. The etiologies were dominated by the sequelae of pulmonary tuberculosis.
However, the inaccessibility of certain diagnostic means made it difficult to systematically search for other aetiologies (especially primary causes) mentioned in the literature. This study is therefore intended to be a prelude to the establishment of a bronchiectasis registry in Burkina Faso in order to improve patient management.
CONFLICT OF INTEREST
The authors declare that they have no potential conflict of interest in relation to the theme of the paper.
REFERENCE
| 1. Brinchault G, Morel V, Meunier C, Belleguic C, Delaval P. Bronchial dilatations. CME - Medicine. 1 Apr 2004;1(2):131-40. |
| 2. Henkle E, Chan B, Curtis JR, Aksamit TR, Daley CL, Winthrop KL. Bronchiectasis patient characteristics and healthcare utilization history in US. Medicare enrollees with prescription drug plans, 2006-2014. Chest 2018; 154: 1311-20. |
| 3. Aksamit TR, O'Donnell AE, Barker A, et al. Adult patients with bronchiectasis: a first look at the US Bronchiectasis Research Registry. Chest 2017; 151: 982–92. |
| 4. Araujo D, Shteinberg M, Aliberti S, et al. The independent contribution of Pseudomonas aeruginosa infection to long-term clinical outcomes in bronchiectasis. Eur Respir J 2018; 51: 1701953. |
| 5. Gao Y-H, Guan W-J, Xu G, et al. The role of viral infection in pulmonary exacerbations of bronchiectasis in adults: a prospective study. Chest 2015 ; 147 : 1635-43. |
| 6. Martinez-Garcia MA, de Gracia J, Vendrell Relat M, et al. Multidimensional approach to non-cystic fibrosis bronchiectasis: the FACED score. Eur Respir J 2014; 43: 1357-67. |
| 7. Meghji J, Simpson H, Squire SB, Mortimer K. A systematic review of the prevalence and pattern of imaging defined post-TB lung disease. PLoS One 2016; 11: e01661176. |
| 8. Meghji J, Banda P, Joekes E, et al. The burden of clinical spirometric and HRCT abnormalities at pulmonary TB treatment completion: a cross-sectional study. Int J Tuberc Lung Dis 2018: 22 (suppl 2): 275-76. |
| 9. Chandrasekaran R, Mac Aogáin M, Chalmers JD, Elborn SJ, Chotirmall SH. Geographic variation in the aetiology, epidemiology and microbiology of bronchiectasis. BMC Pulm Med 2018; 18: 83. |
| 10. Chassagnon G, Brun A-L, Bennani S, Chergui N, Freche G, Revel M-P. Imaging of bronchial dilatations. Rev Pneumol Clin. 2018;74(5):299-314. |
| 11. Gao Y, Guan W, Liu S, Wang L, Cui J, Chen R, et al. Aetiology of bronchiectasis in adults: A systematic literature review. Respirology. 2016; 21(8):1376‑83. |
| 12. Dhar R, Singh S, Talwar D, Mohan M, Tripathi S K, Swarnakar R et al. Bronchiectasis in India: results from the European Multicentre Bronchiectasis Audit and Research Collaboration (EMBARC) and Respiratory Research Network of India Registry. Lancet Glob Health 2019; 7: e1269-79. |
| 13. Ottmani SE, Uplekar MW: Gender and TB: pointers from routine records and reports. Int J Tuberc LungDis 2008,12(7):827-828. |
| 14. Murris-Espin M,, Bassinet L, Le Borgne- Krams A, Boudjema A, Maitre B, Didier A. Dilatations of the bronchi. In : Marchand-Adam S,dir. Evidence-based pneumology. Paris : Margaux Orange ; 2017.p 95-112. |
| 15. Martin C, Regard L, Chassagnon G, Burgel P-R. Etiological diagnosis of bronchial dilatation. Rev Pneumol Clin. 2018;74 (5):292-298. |
| 16. Nachiappan AC, Rahbar K, Shi X, Guy ES, Mortani Barbosa Jr EJ, Shroff GS, et al. Pulmonary tuberculosis: role of radiology in diagnosis and management. Radiographics. 2017;37 (1):52–72. |
| 17. Pasteur MC, Bilton D, Hill AT. British Thoracic Society Bronchiectasis non-CF Guideline Group. British Thoracic Society guideline for non-CF bronchiectasis. Thorax. 2010;65 Suppl 1:i1-58. |
| 18. Redondo M, Keyt H, Dhar R, Chalmers JD. Global impact of bronchiectasis and cystic fibrosis. Breathe. 2016;12(3):222‑35. |
| 19. Ni Y, Shi G, Yu Y, Hao J, Chen T, Song H. Clinical characteristics of patients with chronic obstructive pulmonary disease with comorbid bronchiectasis: a systemic review and meta-analysis. Int J Chron Obstruct Pulmon Dis. 2015;10:1465‑75. |
| 20. Lonni S, Chalmers JD, Goeminne PC, McDonnell MJ, Dimakou K, De Soyza A, et al. Etiology of Non-Cystic Fibrosis Bronchiectasis in Adults and Its Correlation to Disease Severity. Ann Am Thorac Soc. 2015;12 (12):1764-70. |
| 21. Deghdegh K, Boukadoum N, Benali R. Incidence, characteristics and impact of bronchial dilatations (DDB) in COPD patients at CHU Annaba (Algeria). Rev Mal Respiir. 2017;34: A249. |
| 22. Altenburg J, Wortel K, Van der Werf TS, Boersma WG. Non-cystic fibrosis bronchiectasis: clinical presentation, diagnosis and treatment, illustrated by data from a Dutch Teaching Hospital. Neth J Med. 2015; 73 (4):147–54. |
| 23. Aliberti S, Lonni S, Dore S, McDonnell MJ, Goeminne PC, Dimakou K, et al. Clinical phenotypes in adult patients with bronchiectasis. Eur Respir J. 2016; 47(4):1113-22. |
| 24. Martin C, Regard L, Chassagnon G, Burgel P-R. Etiological diagnosis of bronchial dilatation. Rev Pneumol Clin. 2018; 74 (5):292-298. |
FIGURE - TABLES
REFERENCE
| 1. Brinchault G, Morel V, Meunier C, Belleguic C, Delaval P. Bronchial dilatations. CME - Medicine. 1 Apr 2004;1(2):131-40. |
| 2. Henkle E, Chan B, Curtis JR, Aksamit TR, Daley CL, Winthrop KL. Bronchiectasis patient characteristics and healthcare utilization history in US. Medicare enrollees with prescription drug plans, 2006-2014. Chest 2018; 154: 1311-20. |
| 3. Aksamit TR, O'Donnell AE, Barker A, et al. Adult patients with bronchiectasis: a first look at the US Bronchiectasis Research Registry. Chest 2017; 151: 982–92. |
| 4. Araujo D, Shteinberg M, Aliberti S, et al. The independent contribution of Pseudomonas aeruginosa infection to long-term clinical outcomes in bronchiectasis. Eur Respir J 2018; 51: 1701953. |
| 5. Gao Y-H, Guan W-J, Xu G, et al. The role of viral infection in pulmonary exacerbations of bronchiectasis in adults: a prospective study. Chest 2015 ; 147 : 1635-43. |
| 6. Martinez-Garcia MA, de Gracia J, Vendrell Relat M, et al. Multidimensional approach to non-cystic fibrosis bronchiectasis: the FACED score. Eur Respir J 2014; 43: 1357-67. |
| 7. Meghji J, Simpson H, Squire SB, Mortimer K. A systematic review of the prevalence and pattern of imaging defined post-TB lung disease. PLoS One 2016; 11: e01661176. |
| 8. Meghji J, Banda P, Joekes E, et al. The burden of clinical spirometric and HRCT abnormalities at pulmonary TB treatment completion: a cross-sectional study. Int J Tuberc Lung Dis 2018: 22 (suppl 2): 275-76. |
| 9. Chandrasekaran R, Mac Aogáin M, Chalmers JD, Elborn SJ, Chotirmall SH. Geographic variation in the aetiology, epidemiology and microbiology of bronchiectasis. BMC Pulm Med 2018; 18: 83. |
| 10. Chassagnon G, Brun A-L, Bennani S, Chergui N, Freche G, Revel M-P. Imaging of bronchial dilatations. Rev Pneumol Clin. 2018;74(5):299-314. |
| 11. Gao Y, Guan W, Liu S, Wang L, Cui J, Chen R, et al. Aetiology of bronchiectasis in adults: A systematic literature review. Respirology. 2016; 21(8):1376‑83. |
| 12. Dhar R, Singh S, Talwar D, Mohan M, Tripathi S K, Swarnakar R et al. Bronchiectasis in India: results from the European Multicentre Bronchiectasis Audit and Research Collaboration (EMBARC) and Respiratory Research Network of India Registry. Lancet Glob Health 2019; 7: e1269-79. |
| 13. Ottmani SE, Uplekar MW: Gender and TB: pointers from routine records and reports. Int J Tuberc LungDis 2008,12(7):827-828. |
| 14. Murris-Espin M,, Bassinet L, Le Borgne- Krams A, Boudjema A, Maitre B, Didier A. Dilatations of the bronchi. In : Marchand-Adam S,dir. Evidence-based pneumology. Paris : Margaux Orange ; 2017.p 95-112. |
| 15. Martin C, Regard L, Chassagnon G, Burgel P-R. Etiological diagnosis of bronchial dilatation. Rev Pneumol Clin. 2018;74 (5):292-298. |
| 16. Nachiappan AC, Rahbar K, Shi X, Guy ES, Mortani Barbosa Jr EJ, Shroff GS, et al. Pulmonary tuberculosis: role of radiology in diagnosis and management. Radiographics. 2017;37 (1):52–72. |
| 17. Pasteur MC, Bilton D, Hill AT. British Thoracic Society Bronchiectasis non-CF Guideline Group. British Thoracic Society guideline for non-CF bronchiectasis. Thorax. 2010;65 Suppl 1:i1-58. |
| 18. Redondo M, Keyt H, Dhar R, Chalmers JD. Global impact of bronchiectasis and cystic fibrosis. Breathe. 2016;12(3):222‑35. |
| 19. Ni Y, Shi G, Yu Y, Hao J, Chen T, Song H. Clinical characteristics of patients with chronic obstructive pulmonary disease with comorbid bronchiectasis: a systemic review and meta-analysis. Int J Chron Obstruct Pulmon Dis. 2015;10:1465‑75. |
| 20. Lonni S, Chalmers JD, Goeminne PC, McDonnell MJ, Dimakou K, De Soyza A, et al. Etiology of Non-Cystic Fibrosis Bronchiectasis in Adults and Its Correlation to Disease Severity. Ann Am Thorac Soc. 2015;12 (12):1764-70. |
| 21. Deghdegh K, Boukadoum N, Benali R. Incidence, characteristics and impact of bronchial dilatations (DDB) in COPD patients at CHU Annaba (Algeria). Rev Mal Respiir. 2017;34: A249. |
| 22. Altenburg J, Wortel K, Van der Werf TS, Boersma WG. Non-cystic fibrosis bronchiectasis: clinical presentation, diagnosis and treatment, illustrated by data from a Dutch Teaching Hospital. Neth J Med. 2015; 73 (4):147–54. |
| 23. Aliberti S, Lonni S, Dore S, McDonnell MJ, Goeminne PC, Dimakou K, et al. Clinical phenotypes in adult patients with bronchiectasis. Eur Respir J. 2016; 47(4):1113-22. |
| 24. Martin C, Regard L, Chassagnon G, Burgel P-R. Etiological diagnosis of bronchial dilatation. Rev Pneumol Clin. 2018; 74 (5):292-298. |
ARTICLE INFO DOI: 10.12699/jfvpulm.14.41.2023.27
Conflict of Interest
Non
Date of manuscript receiving
15/01/2023
Date of publication after correction
15/06/2023
Article citation
AR. Ouedraogo, D. Zida, G. Bougma, A. Sourabie, A. Ouedraogo, G. Ouedraogo, E Birba, K Boncoungou, G Badoum, M Ouedraogo. Hospital prevalence and etiological profile of bronchiec-tasis in Burkina Faso. J Func Vent Pulm 2023;41(14):27-32