
 English
English
 French
French
Comparison between blood eosinophils in healthy Vietnamese people with the recommended threshold of blood eosinophils in the treatment of chronic obstructive pulmonary disease
Comparaison entre les éosinophiles sanguins chez des personnes vietnamiennes en bonne santé avec le seuil recommandé d'éosinophiles sanguins dans le traitement de la broncho-pulmonaire chronique obstructive
Vinh Nguyen-Nhu1,2 , Thinh Do-Van2 , Son Huynh-Trung2, An Pham-Le2, Sy Duong-Quy1,3
1: Department of Respiratory Functional Exploration, University Medical Center, Ho Chi Minh City, Vietnam
2: University of Medicine and Pharmacy at Ho Chi Minh City, Vietnam
3: Respiratory Department, Lam Dong Medical College, Dalat City, Vietnam
Corresponding author:
Sy Duong-Quy. Respiratory Department. Lam Dong Medical College. Dalat—Vietnam.
E-mail: sduongquy.jfvp@gmail.com
ABSTRACT
Background. Recently, GOLD 2022 recommended inhaled corticosteroid therapy in chronic obstructive pulmonary disease (COPD) patients with blood eosinophils ≥300/uL. However, it is still controversial whether this threshold is applicable in Vietnam. We assessed the potential application of recommended blood eosinophils threshold by GOLD 2020 in the treatment COPD in Vietnam.
Methods: This cross-sectional study was performed on the electronic health records of individuals aged 18 years or older who had attended a medical screening at the University Medical Center of Ho Chi Minh City, between May 1, 2015 to April 30, 2020.
Results. The median number of eosinophils was 140 cells/µL. The mean age of the study sample was 35.51 ± 9.64. Males (48.96%) is approximately equal to females (51.04%). Multivariate logistic regressions revealed that gender, occupation, smoking habit, and place of residence were independent factors associated with over-300 eosinophils in the group of healthy people (p < 0.05).
Conclusion. The recommended GOLD eosinophil threshold of 300 cells/µL could be applied for initiating ICS in Vietnamese people.
KEYWORDS: Eosinophil; Chronic obstructive pulmonary disease; GOLD.
RÉSUMÉ
Introduction. Récemment, GOLD 2022 a recommandé une corticothérapie inhalée chez les patients atteints de maladie pulmonaire obstructive chronique (MPOC) avec des éosinophiles sanguins ≥ 300/uL. Cependant, la question de savoir si ce seuil est applicable au Vietnam reste controversée. Nous avons évalué l'application potentielle du seuil d'éosinophiles sanguins recommandé par GOLD 2020 dans le traitement de la MPOC au Vietnam. Méthodes. Cette étude transversale a été réalisée sur les dossiers de santé électroniques de personnes âgées de 18 ans ou plus ayant participé à un examen médical au Centre médical universitaire de Ho Chi Minh-Ville, entre le 1er mai 2015 et le 30 avril 2020. Résultats. Le nombre médian d'éosinophiles était de 140 cellules/µL. L'âge moyen de l'échantillon de l'étude était de 35,51 ± 9,64. Les hommes (48,96 %) sont à peu près égaux aux femmes (51,04 %). Des régressions logistiques multivariées ont révélé que le sexe, la profession, le tabagisme et le lieu de résidence étaient des facteurs indépendants associés à plus de 300 éosinophiles dans le groupe de personnes en bonne santé (p < 0,05). Conclusion. Le seuil d'éosinophiles GOLD recommandé de 300 cellules/µL pourrait être appliqué pour initier un CSI chez les Vietnamiens.
MOTS CLÉS: Éosinophile; Broncho-pulmonaire chronique obstructive; GOLD.
INTRODUCTION
According to GOLD (GLOBAL STRATEGY FOR PREVENTION, DIAGNOSIS AND MANAGEMENT OF COPD) 2020, an exacerbation of COPD (chronic obstructive pulmonary disease) is defined as "an acute worsening of respiratory symptoms that warrants additional medication" [1].
COPD exacerbations are associated with more airway inflammation, rapid decline in respiratory function, poor quality of life, increased mortality and ultimately increased socio-economic costs [2].
COPD is a diagnosis that includes many different pathological phenotypes [1].
Therefore, manifestations of COPD exacerbations and treatment responses can vary among patients. Some exacerbations are associated with airway inflammation caused by neutrophils, while others are associated with eosinophilic-driven inflammation [3].
According to GOLD, a blood eosinophil count threshold of more than 300 cells/μl can be used to identify patients most likely to benefit from inhaled corticosteroid (ICS) treatment.
However, the typical range of eosinophil counts in the general population is not well established and there are not many studies evaluating eosinophil counts in healthy adults in Vietnam.
The threshold of 300 cells/μl is often suspected because it is thought to be within the normal range of blood eosinophil counts in Vietnamese due to the role of potential confounding factors other than allergies, such as age, sex, environmental exposure, or the presence of unclear diseases.
Therefore, applying this threshold for ICS indication in the maintenance of stable COPD has not been straightforward yet.
As a result, we aimed to investigate the blood eosinophils count in healthy Vietnamese people and assessed the applicability of the GOLD 2020 eosinophil count threshold for COPD in Vietnam.
METHODS
This was a descriptive, retrospective, and analytical cross-sectional study.
Our inclusion criteria were based on the electronic health records of those came for routine medical check at the Medical Examination Department and the Health Examination Department at the request at UMC, which were healthy people 18 years of age or older, without any health problems recorded at the time of examination, within 5 years from 01 May, 2015 to 30 April, 2020.
Exclusion criteria were records of people with any medical conditions, incomplete records, or pregnant women.
We collected baseline characteristics, record number, eosinophil count, smoking habits, education, parasite test results.
Data were divided into subgroups according to the predefined groups of eosinophils count: under 100 cells/µL, 100-300 cells/µL, and above 300 cells/µL. The distribution of cases across demographic groups were also investigated.
We calculated percentages for binary, nominal and ordinal variables, and mean, standard deviation and median for continuous variables.
The difference between ratios for categorical variables was assessed using Chi-square test or Fisher's exact test.
The independent T test or Mann-Whitney test was used to compare the mean or median of two continuous variables for the variable with or without a normal distribution.
Kolmogorov-Smirnov test was used to verify the distribution of eosinophils. We performed logistic regression analyses to evaluate the association between demographic characteristics and the blood eosinophils count.
Bayesian Model Averaging (BMA) was used to choose the optimal variables to be included in the regression model.
A p-value of < 0.05 was considered statistically significant. All analyses were performed using S.P.S.S 20 software.
RESULTS
Within 5 years, 1203 applications were included and 47 applications did not meet the inclusion criteria.
Population demographics
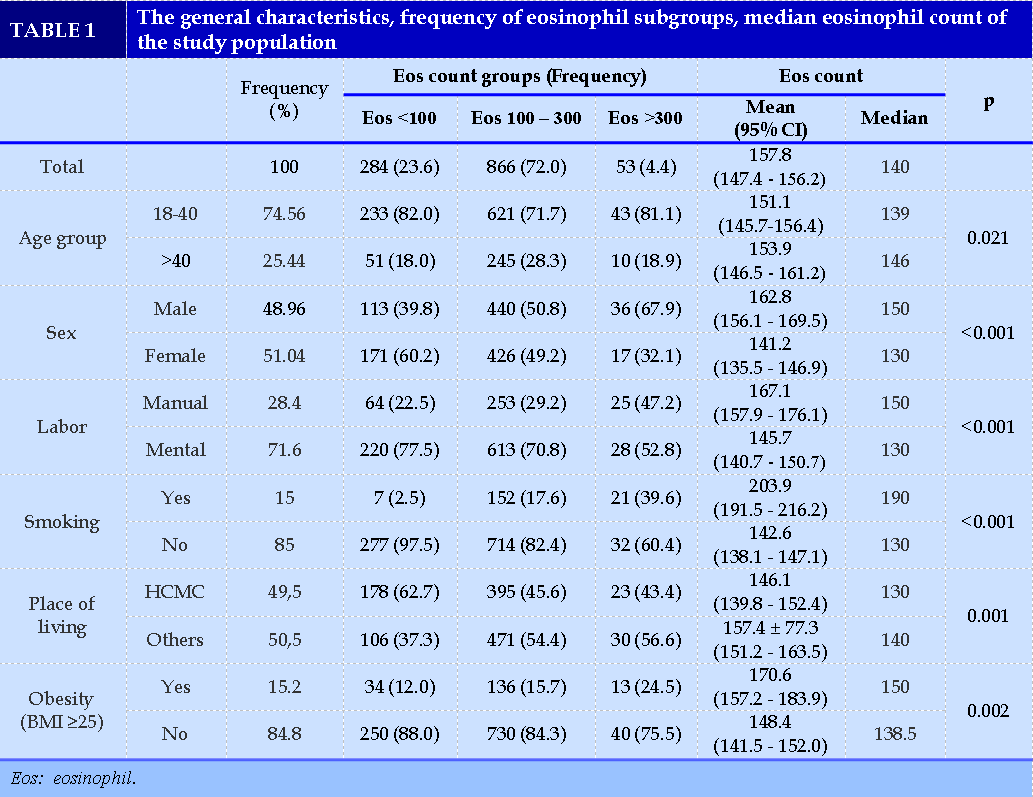
In our study population, the mean age was 35.51 ± 9.64, and nearly 75% fell into the 18-40 age group. Males (48.96%) were approximately equal to females (51.04%).
Manual labor accounted for 28,4%, and 15% of the study sample were smokers. 50,72% had overweight or obese, and 49.5% lived in Ho Chi Minh City (HCMC) (Table 1).
Distribution of eosinophils across the demographic groups
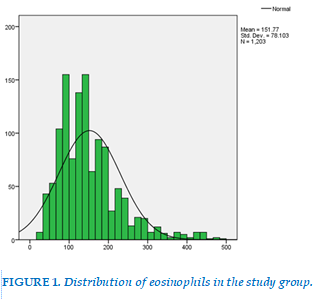
The eosinophils count did not conform to normal distribution (Kolmogorov-Smirnov test, p<0.001). The median value was 140 cells/µL, IQR 100 – 190 cells/µL (Figure 1).
People having blood eosinophils count less than 100 cells/µL, from 100 to 300 cells/µL and higher than 300 cells/µL accounted for 23.6%, 72.0% and 4.4%, respectively.
The median values of the following group: aged over 40, male gender, manual labor, smoker, not living in Ho Chi Minh City, and obesity, were all higher than those of the respective counterpart groups (p < 0.05).
Factors associated with blood eosinophil count higher than 300 cells/µL
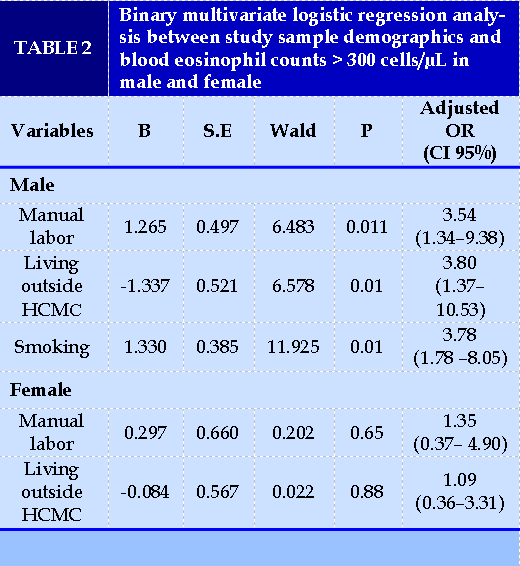
We used Bayesian Model Averaging (BMA) to choose the optimal variables to be included in the regression model. BMA showed that the model containing labor group, smoking and place of living had the lowest BIC and the highest post probability.
The regression model from those 3 variables indicated that in men, manual labor, smoking and living outside Ho Chi Minh City had the association with blood eosinophils count higher than 300 cells/µL. The accuracy of the predicted result was 93.9%. In women, we did not find any association between those factors with blood eosinophils count (Table 2).
DISCUSSION
Demographics
The mean age of our study group was 35.51 ± 9.64, whereas, in the LEAD study, the mean age was 41.8 years old [4]. This difference may be due to the older population of Austria and Western countries compared to Vietnam. In addition, the Western primary care system may be better, so the proportion of the healthy population over 40 years old may be higher. Vietnamese people do not really appreciate regular health check-ups. Therefore, older people are more prone to chronic diseases and have a tendency to visit specialty clinics rather than screening clinics.
In our study, only 15.2% of the sample have obesity according to WHO criteria for Asian people and 15% are smokers, mainly manual labor in both genders. That may imply an improvement in people’s awareness of health over time.
Distribution of eosinophils count
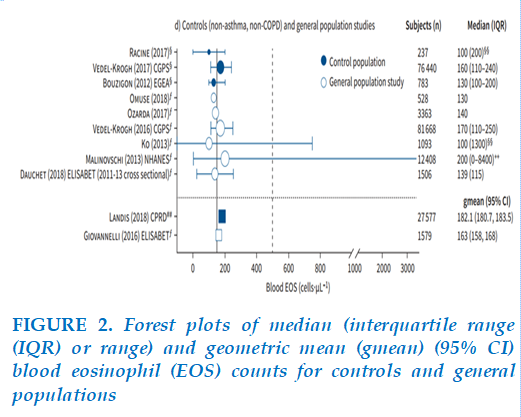
Our study showed widely dispersed and non-normally distributed values of blood eosinophils count and a high value of standard deviation (Figure 1). Blood eosinophils count demonstrated a right-skewed distribution profile and showed consistency with the general trendline of several previous studies. Thus, the median rather than the mean was used for statistical calculation. Our median blood eosinophils count was 140 cells/µL, which was less than the mean (157.8 cells/µL). In many general population studies, six revealed median counts <150 cells/µL, one large study reported a count of 170 cells/µL, and the National Health and Nutrition Examination Survey recorded a count of 200 cells/µL (Figure 2) [5,15].
Our median count was consistent with most research findings and supported the applicability of the GOLD eosinophil ICS treatment threshold in Vietnamese COPD patients.
Factors associated with the blood eosinophil count higher than 300 cells/µL
Using univariate analysis, we found that the median eosinophils counts of the following categories were higher than the counterpart groups: age above 40, male sex, manual labor, smoking, living outside HCM city, and obesity (p<0.05). For variable selection in the regression model, Bayesian model averaging (BMA) was employed, and only three variables were considered: types of labor, smoking, and place of living. The model containing those three variables was able to predict high eosinophil count in men but not in women (Table 2). This could be attributed to the interaction of types of labor and smoking: men manual laborers are more likely to smoke than women. Gender, age and BMI were excluded from the predictive model.
Male sex and obesity were found to be independent factors associated with blood eosinophil counts >210 cells/µL in some studies, potentially due to the effect of intrinsic estrogen [6]. However, these variables were not chosen for regression analysis by BMA approach in our study, probably because of insufficient sample size and different eosinophils cut-offs. Because of the potential influences on blood eosinophil counts of unknown biological and environmental factors related to gender that were not accounted for, we performed logistic regression in separate gender groups. In men, manual labor, smoking and living outside of HCM City had a significant association with blood eosinophils count > 300 cells/µL. The fact that blood eosinophil counts are higher in children was already recognized; thus, we did not include people younger than 18 years old in our study. The LEAD study also showed that blood eosinophil counts were irrelevant of age after 18 in both genders [7], which was consistent with our results.
Current smoking was proven to be significantly associated with the increase of not only eosinophils but also all other types of leucocytes, including neutrophils, lymphocytes, monocytes, and basophils [1]. Our result revealed that in smokers, the median blood eosinophils count was 190.00 cells/µL compared to 130.00 cells/µL in non-smokers. Current smoking was linked to blood eosinophils count > 300 cells/µL with the OR=3.78 (95% CI: 1.78 – 8.05). In over 100.000 participants of LEAD study [4], the mean blood eosinophil count in smokers versus non-smokers was 252 cells/µL versus 149 cells/µL (p = 0.0093). Our finding was also consistent with Higuchi T’s result [8], which revealed that smoking was significantly associated with high blood eosinophil count. Cigarette smoke can increase T lymphocytes count, mainly CD8, subsequently leading to increased production of interleukin-5 (IL-5), a chemical that activates other cytokines and increases eosinophils [9].
Furthermore, in non-smokers who develop COPD with eosinophilic inflammation, genetic factors may play a role in increasing CD8 cells, according to O'Shaughnessy [10].
Place of living can be a confounding factor in blood eosinophil count. We found that in men, living outside HCM City was associated with high blood eosinophil count (OR=3.80). This can be explained by the difference in sanitary regions, which predisposes rural populations to parasitic infection, as well as exposure to air pollution from industrial zones and heavy traffics, which can induce an inflammation cascade comparable to the inflammation pathways in smokers.
Regarding obesity, The LEAD study 4 concluded that the obese group was significantly associated with a high blood eosinophil count. Adipose tissue may be one of the major sources of extracellular estrogen derived from metabolizing androgen precursors [11] resulting in increased circulating estrogen levels in obese subjects [12].
Estrogen may also act in synergy with the hormone leptin secreted by adipocytes which has been shown to correlate with body fat rate and BMI [12]. It has been reported that estrogen has anti-inflammatory effects and lowers TNF-α production and the action of interleukin-5 by competitively binding to receptors on eosinophils, thus reducing peripheral blood eosinophil concentrations [13]. However, the BMA computation approach returned zero inclusion probability on the BMI and was excluded from the model.
Applicability of GOLD eosinophils threshold in the treatment of COPD patients in Vietnam
Using a GOLD-recommended cut-off point (>300 cells/µL) is useful in the initial treatment of ICS in COPD. As there are cases of eosinophils that have
not yet reached the threshold (300 cells/µL), it is necessary to consider eosinophil level as a continuous variable. However, the observed clinical course of leukocytosis suggests that the choice to commence ICS should be made with setting considerations in mind. Thus, independent factors associated with eosinophil prediction above 300 cells/µL would assist in the personalized treatment of COPD patients.
Eosinophil count fluctuations from one subtype to another were more prevalent in subjects with eosinophil counts close to the cutoff value (100 cells/µL, 300 cells). /µL). GOLD 2020 uses thresholds of > 300 cells/µL and <100 cells/µL to identify individuals with a higher and lower probability, respectively, of benefiting from ICS treatment [1]. The GOLD 2020 revision states that these are "estimates, rather than exact cut-off values" [1]. The purpose of this recommendation is to emphasize that small changes in blood eosinophil counts do not dictate a complete change in ICS treatment, even when the change fluctuates around a threshold value. If the eosinophil count rises from less than 300 to greater than 300 cells/µL, it must be consistent with the clinical course and the influence of toxicologically related promoters.
Occupational group, smoking habit, and place of residence were independent factors associated with eosinophils over 300 in normal healthy subjects (p < 0.05) with a predicted mean of 93 %. As a result, if these subjects develop COPD in the future, it is expected that their eosinophilic phenotype is likely to become more common.
Implications for COPD management
In the current COPD treatment practice, doctors are concerned that Vietnamese patients are living in countries with poor sanitary conditions and high rates of parasitic infections so BCAT levels will be higher than in other countries. This will lead to the application of the BCAT index to guide the use of ICS, which will lead to overuse. As an increased incidence of ICS-associated pneumonia has been observed in several clinical trials [12]. Prolonged use of ICS can cause systemic adverse events that have been demonstrated in studies, such as adrenal suppression, osteoporosis, and diabetes [14].
Our study proved that the BCAT of healthy Vietnamese people was not as high as common belief. Therefore, the application of BCAT in the management of COPD is still appropriate .However, to answer the question of whether the eosinophil index can be utilized for patients with COPD, a study description of BCAT in COPD should be undertaken to supplement this study as many Vietnamese individuals have eosinophil levels below 300 cells/µL.
The 300 cells/μL threshold for ICS treatment in patients with COPD is sometimes suspected since it is assumed to be within the normal range of blood eosinophil levels (≤500 cells/µL).
In the healthy population, we observed that blood eosinophil counts were consistently higher in men than in women regardless of age. In addition, mean eosinophil levels were much lower than the GOLD threshold of 300 cells/µL.
Factors independently related to the predictability of eosinophil counts of more than 300 cells/µL in our population sample included smoking habits, place of residence, and possible occupational group, which may affect the predictive role of ICS treatment in some groups of COPD patients in Vietnam.
CONFLICT OF INTEREST
CONCLUSIONS
The median value of the eosinophil count among our study population was 140. Factors affecting eosinophils were age group, gender, occupational group, smoking habit, place of residence and BMI.
Although our findings cannot be generalized to the whole of Vietnam as well as the causal relationship was not determined, it is suggested that the recommended GOLD threshold for ICS initiation of 300 cells/µL in Vietnamese could be applied.
REFERENCES
| 1. Halpin DMG, Criner GJ, Papi A, et al. Global Initiative for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease. The 2020 GOLD Science Committee Report on COVID-19 and Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med. Jan 1 2021;203(1):24-36. doi:10.1164/rccm.202009-3533SO. |
| 2. Kovalszki A, Weller PF. Eosinophilia. Prim Care. Dec 2016;43(4):607-617. doi:10.1016/j.pop.2016.07.010. |
| 3. White AJ, Gompertz S, Stockley RA. Chronic obstructive pulmonary disease . 6: The aetiology of exacerbations of chronic obstructive pulmonary disease. Thorax. Jan 2003;58(1):73-80. doi:10.1136/thorax.58.1.73 |
| 4. Hartl S, Breyer MK, Burghuber OC, et al. Blood eosinophil count in the general population: typical values and potential confounders. Eur Respir J. May 2020;55(5)doi:10.1183/13993003.01874-2019 |
| 5. Vedel-Krogh S, Nielsen SF, Lange P, Vestbo J, Nordestgaard BG. Blood Eosinophils and Exacerbations in Chronic Obstructive Pulmonary Disease. The Copenhagen General Population Study. Am J Respir Crit Care Med. May 1 2016;193(9):965-74. doi:10.1164/rccm.201509-1869OC |
| 6. Landis S, Suruki R, Maskell J, Bonar K, Hilton E, Compton C. Demographic and Clinical Characteristics of COPD Patients at Different Blood Eosinophil Levels in the UK Clinical Practice Research Datalink. Copd. Apr 2018;15(2):177-184. doi:10.1080/15412555.2018.1441275. |
| 7. DiSantostefano RL, Hinds D, Le HV, Barnes NC. Relationship between blood eosinophils and clinical characteristics in a cross-sectional study of a US population-based COPD cohort. Respir Med. Mar 2016;112:88-96. doi:10.1016/j.rmed.2016.01.013 |
| 8. Higuchi T, Omata F, Tsuchihashi K, Higashioka K, Koyamada R, Okada S. Current cigarette smoking is a reversible cause of elevated white blood cell count: Cross-sectional and longitudinal studies. Prev Med Rep. Dec 2016;4:417-22. doi:10.1016/j.pmedr.2016.08.009. |
| 9. Coyle AJ, Erard F, Bertrand C, Walti S, Pircher H, Le Gros G. Virus-specific CD8+ cells can switch to interleukin 5 production and induce airway eosinophilia. J Exp Med. Mar 1 1995;181(3):1229-33. doi:10.1084/jem.181.3.1229. |
| 10. O'Shaughnessy TC, Ansari TW, Barnes NC, Jeffery PK. Inflammation in bronchial biopsies of subjects with chronic bronchitis: inverse relationship of CD8+ T lymphocytes with FEV1. Am J Respir Crit Care Med. Mar 1997;155(3):852-7. doi:10.1164/ajrccm.155.3.9117016. |
| 11. Rao GN. Influence of diet on tumors of hormonal tissues. Prog Clin Biol Res. 1996;394:41-56. |
| 12. Mantzoros CS. The role of leptin and hypothalamic neuropeptides in energy homeostasis: update on leptin in obesity. Growth Horm IGF Res. Jun 2001;11 Suppl A:S85-9. doi:10.1016/s1096-6374(01)80014-9. |
| 13. Peterson AP, Altman LC, Hill JS, Gosney K, Kadin ME. Glucocorticoid receptors in normal human eosinophils: comparison with neutrophils. J Allergy Clin Immunol. Sep 1981;68(3):212-7. doi:10.1016/0091-6749(81)90186-x. |
| 14. Pandya D, Puttanna A, Balagopal V. Systemic effects of inhaled corticosteroids: an overview. Open Respir Med J. 2014;8:59-65. doi:10.2174/1874306401408010059. |
| 15. Benson VS, Hartl S, Barnes N, et al. Blood eosinophil counts in the general population and airways disease: a comprehensive review and meta-analysis. Eur Respir J 2022; 59: 2004590 [DOI: 10.1183/13993003.04590-2020] |
FIGURE - TABLES
REFERENCES
| 1. Halpin DMG, Criner GJ, Papi A, et al. Global Initiative for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease. The 2020 GOLD Science Committee Report on COVID-19 and Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med. Jan 1 2021;203(1):24-36. doi:10.1164/rccm.202009-3533SO. |
| 2. Kovalszki A, Weller PF. Eosinophilia. Prim Care. Dec 2016;43(4):607-617. doi:10.1016/j.pop.2016.07.010. |
| 3. White AJ, Gompertz S, Stockley RA. Chronic obstructive pulmonary disease . 6: The aetiology of exacerbations of chronic obstructive pulmonary disease. Thorax. Jan 2003;58(1):73-80. doi:10.1136/thorax.58.1.73 |
| 4. Hartl S, Breyer MK, Burghuber OC, et al. Blood eosinophil count in the general population: typical values and potential confounders. Eur Respir J. May 2020;55(5)doi:10.1183/13993003.01874-2019 |
| 5. Vedel-Krogh S, Nielsen SF, Lange P, Vestbo J, Nordestgaard BG. Blood Eosinophils and Exacerbations in Chronic Obstructive Pulmonary Disease. The Copenhagen General Population Study. Am J Respir Crit Care Med. May 1 2016;193(9):965-74. doi:10.1164/rccm.201509-1869OC |
| 6. Landis S, Suruki R, Maskell J, Bonar K, Hilton E, Compton C. Demographic and Clinical Characteristics of COPD Patients at Different Blood Eosinophil Levels in the UK Clinical Practice Research Datalink. Copd. Apr 2018;15(2):177-184. doi:10.1080/15412555.2018.1441275. |
| 7. DiSantostefano RL, Hinds D, Le HV, Barnes NC. Relationship between blood eosinophils and clinical characteristics in a cross-sectional study of a US population-based COPD cohort. Respir Med. Mar 2016;112:88-96. doi:10.1016/j.rmed.2016.01.013 |
| 8. Higuchi T, Omata F, Tsuchihashi K, Higashioka K, Koyamada R, Okada S. Current cigarette smoking is a reversible cause of elevated white blood cell count: Cross-sectional and longitudinal studies. Prev Med Rep. Dec 2016;4:417-22. doi:10.1016/j.pmedr.2016.08.009. |
| 9. Coyle AJ, Erard F, Bertrand C, Walti S, Pircher H, Le Gros G. Virus-specific CD8+ cells can switch to interleukin 5 production and induce airway eosinophilia. J Exp Med. Mar 1 1995;181(3):1229-33. doi:10.1084/jem.181.3.1229. |
| 10. O'Shaughnessy TC, Ansari TW, Barnes NC, Jeffery PK. Inflammation in bronchial biopsies of subjects with chronic bronchitis: inverse relationship of CD8+ T lymphocytes with FEV1. Am J Respir Crit Care Med. Mar 1997;155(3):852-7. doi:10.1164/ajrccm.155.3.9117016. |
| 11. Rao GN. Influence of diet on tumors of hormonal tissues. Prog Clin Biol Res. 1996;394:41-56. |
| 12. Mantzoros CS. The role of leptin and hypothalamic neuropeptides in energy homeostasis: update on leptin in obesity. Growth Horm IGF Res. Jun 2001;11 Suppl A:S85-9. doi:10.1016/s1096-6374(01)80014-9. |
| 13. Peterson AP, Altman LC, Hill JS, Gosney K, Kadin ME. Glucocorticoid receptors in normal human eosinophils: comparison with neutrophils. J Allergy Clin Immunol. Sep 1981;68(3):212-7. doi:10.1016/0091-6749(81)90186-x. |
| 14. Pandya D, Puttanna A, Balagopal V. Systemic effects of inhaled corticosteroids: an overview. Open Respir Med J. 2014;8:59-65. doi:10.2174/1874306401408010059. |
| 15. Benson VS, Hartl S, Barnes N, et al. Blood eosinophil counts in the general population and airways disease: a comprehensive review and meta-analysis. Eur Respir J 2022; 59: 2004590 [DOI: 10.1183/13993003.04590-2020] |
ARTICLE INFO DOI: 10.12699/jfvpulm.14.42.2022.33
Conflict of Interest
Non
Date of manuscript receiving
15/01/2023
Date of publication after correction
15/6/2023
Article citation
Nguyen-Nhu V, Do-VanT, Huynh-Trung S, Pham-Le A, Duong-Quy S. Comparison between blood eosinophils in healthy Vietnamese people with the recommended threshold of blood eosinophils in the treatment of chronic obstructive pulmonary disease. J Func Vent Pulm 2023;42(14):33-38