
 English
English
 French
French
Serum Procalcitonin Levels And Association With Blood Culture Results At General Hospital Ninh Thuan Province In 2021
Niveaux de procalcitonine sérique et association avec les résultats d'hémoculture à l'hôpital général Province de Ninh Thuan en 2021
Le Huy Thach1, Le Van Thanh1, Do Thuy Dung1, Le Quoc Thang1
1: Ninh Thuan General Hospital
Corresponding author:
Le Huy Thach. Ninh Thuan General Hospital. Hanoi, Vietnam.
E-mail: lh.thach67@gmail.com
ABSTRACT
Introduction. Procalcitonin (PCT), regarded as a biomarker specific for bacterial infections.
Objective. Determination of serum PCT concentration in patients positive blood culture sepsis and describe the relationship between serum PCT concentration with isolated bacteria.
Subjects and Methods. Descriptive cross-sectional study on 480 patients diagnosed with sepsis, at Ninh Thuan province general hospital from January-September 2021.
Results. Median serum PCT concentration in the positive blood culture group (15.6 ng/ml), higher than in the negative blood culture group (p<0.05). The area under the curve was 0. 83 (p<0.001) high or low PCT concentrations were able to identify positive blood culture sepsis with a cut-off of 0. 4 ng/ml with a sensitivity of 80% and a specificity of 93%. The median serum PCT concentration of the gram-negative bacteria group (32. 6 ng/ml) was higher than that of the gram-positive group (p<0.05).
Conclusions. PCT can distinguish positive blood culture sepsis, as well as between different bacterial species.
KEYWORDS: Sepsis; Procalcitonin; Positive blood culture
RÉSUMÉ
Introduction. Procalcitonine (PCT), considérée comme un biomarqueur spécifique des infections bactériennes.
Objectif. Détermination de la concentration sérique de PCT chez les patients présentant une septicémie positive à l'hémoculture et décrire la relation entre la concentration sérique de PCT et les bactéries isolées.
Sujets et méthodes. Étude transversale descriptive sur 480 patients diagnostiqués avec une septicémie, à l'hôpital général de la province de Ninh Thuan de janvier à septembre 2021.
Résultats. Concentration médiane de PCT sérique dans le groupe hémoculture positive (15,6 ng/ml), supérieure à celle du groupe hémoculture négative (p<0,05). L'aire sous la courbe était de 0,83 (p<0,001) des concentrations élevées ou basses de PCT ont permis d'identifier une septicémie positive d'hémoculture avec un seuil de 0,4 ng/ml avec une sensibilité de 80 % et une spécificité de 93 %. La concentration sérique médiane de PCT du groupe de bactéries gram-négatives (32,6 ng/ml) était supérieure à celle du groupe gram-positif (p<0,05).
Conclusions. La PCT peut distinguer une septicémie positive à l'hémoculture, ainsi qu'entre différentes espèces bactériennes.
MOTS CLÉS: État septique; procalcitonine; Hémoculture positive.
INTRODUCTION
Sepsis (NTH) is a life-threatening condition that can result from infection. In 2017, an estimated 48.9 million NTH cases were recorded worldwide and 11 million NTH-related deaths were reported, accounting for 19.7% of all deaths globally [15]. Blood cultures are considered the gold standard for identifying NTH, and it is possible to identify pathogens and then test for sensitivity to antibiotics, however results are slow (after 3-7 days) [2] and not always positive. Therefore, the study of biomarkers for sepsis is an area of interest and research. Procalcitonin (PCT) is one of the markers that has shown great promise in identifying NTH, assessing disease severity, and guiding antibiotic surveillance [8], [16], [19].
Procalcitonin is a peptide precursor of calcitonin and is part of the inflammatory process in sepsis. PCT concentrations tend to be elevated in bacterial infections [16] and high PCT has been known to predict sepsis [13]. PCT is detectable in serum within 4 hours and has a half-life of 22-26 hours. Peaks occur between 12-48 hours [7], [9], [12].
So far, there are many authors in the world as well as in Vietnam studying PCT in infectious diseases. Le Phan Ngoc Bich (2018), showed an association between increased PCT with early neonatal infection and mean PCT levels in pediatric patients with early neonatal infections of 4,176 ± 15,552 ng/ml [1]. However, this study has not shown PCT levels in positive blood cultures as well as the association between pathogenic bacteria (VK). Ninh Thuan General Hospital has long implemented PCT tests and blood cultures but there has been no research on this issue. Stemming from that fact, in order to contribute to improving the quality of treatment and limiting mortality due to NTH, we have implemented the topic " Serum procalcitonin levels and their association with blood culture results at Ninh Thuan General Hospital in 2021" with the following two goals:
Determination of serum procalcitonin levels in patients with positive blood culture sepsis.
Describe the association of serum procalcitonin levels and isolated pathogenic bacterial agents.
RESEARCH SUBJECTS AND METHODS
Subjects of study
A total of 480 patients diagnosed with NTH were prescribed PCT tests from January 1 to September 9, 2021.
Selection criteria. patients diagnosed with NTH are prescribed PCT tests and blood cultures at the same time.
Exclusion criteria. Only for PCT tests but not for blood cultures.
Research Methodology
Study design: Ccross-description.
Sample size and how to choose a template: Sample size and convenient template selection.
Data collection method
How to collect data. Patients who meet the sample selection criteria are included in the research group to record information such as age, gender, number of admission records, diagnosis, PCT test results and blood culture.
Evaluation techniques and standards
PCT: Take 1.5 ml of venous blood into a tube without EDTA antifreeze (red cap tube). Readings on the Cobas E411 automatic immunocomputer. Assessment of PCT increase according to Meisner (2014) includes [12]: Normal (< 0.05 ng/mL); Slight increase (0.05<PCT<2 ng/mL); Moderate increase (2≤PCT<10 ng/mL); Elevated (PCT>10 ng/mL).
- Blood culture: Blood collection before using antibiotics. Take 3-5 ml of venous blood, ensure the correct sterile technique, put it in a Bact/Alert FA plus blood culture bottle. Isolation of pathogenic VK according to the guidance of the Ministry of Health (2017), identification of VK using the Phoenix 100 automatic system.
Data processing and analysis
Data is entered and processed using SPSS software. Qualitative variables are represented as ratios (%). Use median values and percentile intervals (25 and 75) to describe quantitative variables that do not have a standard distribution.
RESULTS
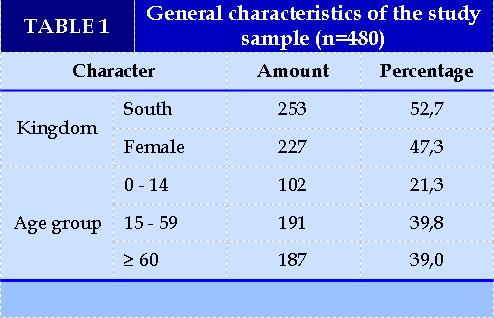
Serum PCT levels in patients with sepsis (Table 1)
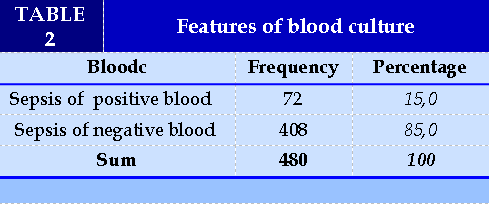
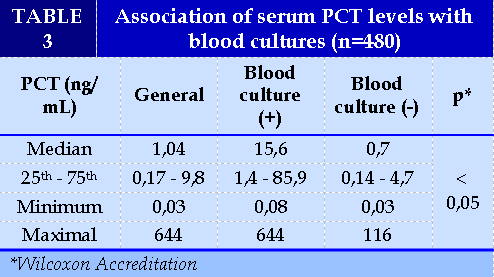
Nam and female are equivalent with a male:female ratio of 1.1:1. The distribution by age group is quite similar, highest in the 15-59 year old group and lowest in the 0-14 year old group. (Table 2). Sepsis with positive blood results accounted for 15.0% (Table 3). The median serum PCT concentration in the positive blood culture group was 15.6 ng/ml higher than in the negative blood culture group (0.7 ng/ml), which was statistically significant (p<0.05).(Table 4).
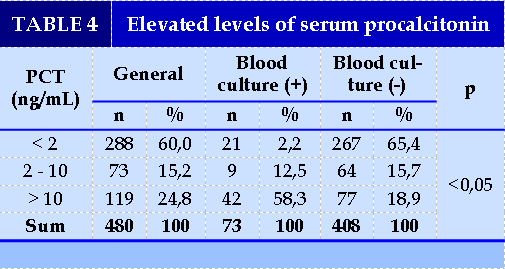
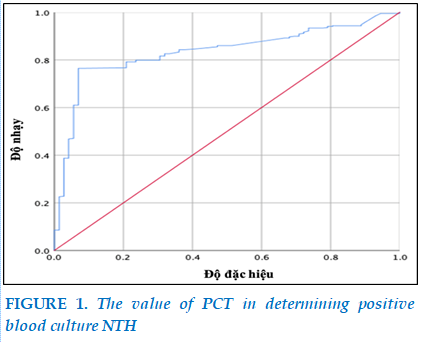
The majority of PCT concentrations at < 2 ng / nl accounted for 60.0%, followed by >10 ng / ml accounted for 24.8%. There were significant differences between PCT and blood cultures (p<0.05). (Figure 1).
The area below the curve of 0.83 with p<0.001 is statistically significant, so high or low PCT concentrations are capable of identifying positive blood cultures with a cut-off point of 0.4 ng/ml, 80% sensitivity and 93% specificity .
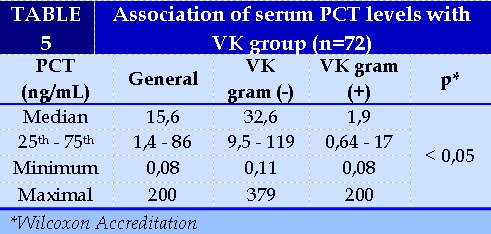
Association of serum PCT levels with pathogenic bacteria (Table 5)
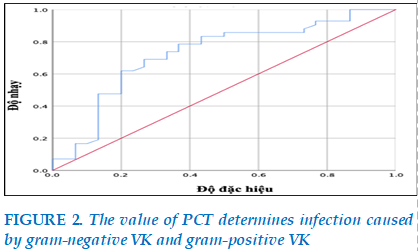
The serum PCT median concentration of gram-negative blood cultures was 32.6 ng/ml, higher than that of the gram-positive VK group (p<0.05). (Figure 2)
The area below the curve of 0.72 with p<0.01 is statistically significant, so high or low PCT concentrations are capable of identifying NTH due to gram-negative VK with a cut-off point of 10.2 ng/ml, a sensitivity of 74% and a specificity of 67%. (Table 6).
In the gram-negative VK group, E. coli accounted for 50.0% and in the gram-positive VK group Staphylococcus coagulase (-) accounted for 54.8%. The median concentration of PCT in gram-negative VKs is much higher than in gram-positive VKs.
DISCUSSION
Serum PCT levels in patients with sepsis
The results of the study in Table 2 showed that sepsis with positive blood results accounted for 15.0%, similar to Kalathia, positive blood cultures accounted for 17.8% [11], Le Huy Thach, positive blood cultures in early neonatal infections accounted for 15.3% [17].
On the association of serum PCT levels and blood culture results; the results in Table 3 showed that the median concentration of positive blood culture group serum PCT was higher than that of negative blood culture group (p<0.05). This suggests that PCT is very valuable in early prediction of blood culture outcomes for conducting treatment interventions. Similar to us, Nguyen Viet Phuong et al. (2017), the average pct concentration of group BN with positive blood culture sepsis was 21.76 ± 36.62 ng / ml, which is statistically significant higher than that of negative blood culture sepsis group. In addition, this study also showed that PCT concentrations > 10 ng/ml accounted for the highest proportion (55.5%) [6], similar to ours (58.3%) and at the same time there was also a significant difference between the levels of increased PCT between the 2 groups of positive and negative blood cultures [6]. Research by Yan et al. (2017) on PCT levels in NTH caused by different bacterial sources and species, resulted in 486 sepsis positive for blood cultures, 254 (52.26%) positive for Gram-negative bacteria and 202 (42.18%) positive for Gram-positive bacteria. Median PCT concentrations in the gram-negative group were higher than in the gram-positive group, statistically significant differences with p<0.001 [20].
For the PCT cut-off point for determining a positive blood culture NTH, our study showed that an area below the curve of 0.83 with p<0.001 is statistically significant, so high or low PCT concentrations are capable of identifying a positive blood culture NTH with a cut-off point of 0.4 ng/ml with an 80% sensitivity and 93% specificity. Bui Thi Hong Chau et al. (2010) studied 132 patients with severe infection, the best cut-off point of PCT in the diagnosis of severe infection was 1.77 ng/mL, with a sensitivity of 78.79%, specificity of 73.33%, positive predictive value of 86.6% and negative predictive value of 38.89% [3].
When we analyzed the association of serum PCT levels and isolated pathogenic bacteria, we found that in patients with gram-negative VK infection, there were higher serum PCT levels than in gram-positive VK infection. Specifically, the results in table 5 showed that the serum PCT median concentration of the gram-negative VK-positive blood culture group was 32.6 ng/ml, higher than that of the gram-positive VK group (p<0.05). Our results are similar to other studies around the world. Yan et al. (2017), median PCT concentrations in the gram-negative VK-positive group were higher than in the gram-positive VK-positive group (p<0.001), In addition, this study showed that in NTH patients, the cut-off point of PCT of 10.3 ng/ml could identify infections caused by gram-negative VK, with a specificity of 80.2% [20]. Similarly, research by Yunus et al. (2018), on the use of PCT to determine the severity of NTH, outcome of BN, and characteristics of infection, showed that sites that are commonly infected with gram-negative VK have higher PCT values than sites infected with gram-positive VK (p=0.03). The sensitivity and specificity of PCT for septic shock were 63% and 65% [21], respectively.
Regarding the proportion of isolated bacterial pathogens, the results showed that in the gram-negative VK group, E. coli accounted for 50.0%, and in the gram-positive VK group Staphylococcus coagulase (-) accounted for 54.8%. Comparison of isolated bacterial models in blood specimens, showed that there were large differences depending on the subject and region studied. Japoni (2009) gram-positive bacteria accounted for the majority of positive cases, with Staphylococcus coagulase (-) accounting for 66.7% and S. aureus accounting for only 1.5%. Among gram-negative bacteria, the highest proportion is Acinetobacter (10.6%), E. coli accounts for only 3% [10]. The study, authored by Velaphi et al. (2019), showed that the cause of early-onset neonatal sepsis was group B Streptococcus accounting for 35.4%, followed by Streptococci viridans (24.2%), S. aureus accounting for only 3%. Among gram-negative bacteria, VK with the highest pathogenicity rate was E. coli (8.1%), followed by A. baumannii at 4.0% [18].
Meanwhile, our results are consistent with opota's
2015) study, where gram-negative VK agents account for 62% and gram-positive VK is 35.4%. Of the gram-negative VK group, the most common is E. coli (28.6%) [14]. Cao Minh Nga (2009), in the group of gram-negative VK causing sepsis accounted for the highest proportion of E. coli (24.11%) and Klebsiella spp. (17.85%). In the gram-positive VK group is S. aureus (8.93%) [4]. Tran Thi Thanh Nga et al. (2015), common causative agents of sepsis are E. coli (20.6%), S. aureus (18.5%), Klebsiella (8.9%), A. baumannii (8%), S. maltophilia (6.8%), Negative Staphylococcus coagulase (5.9%), B. pseudomallei (4.4%) and P. aeruginosa (4%) [5] . With the aforementioned results, it can be seen that E. coli has emerged as the leading VK causing sepsis in the pathogenic VK model at BVs.
CONCLUSION
Sepsis with positive blood cultures accounted for 15.0%. The median concentration of serum PCT in the positive blood culture group was higher than in the negative blood culture group (p<0.05). The area below the curve is 0.83 (p<0.001), high or low PCT concentrations are capable of identifying a positive blood culture NTH with a cut-off point of 0.4 ng/ml, a sensitivity of 77% and a specificity of 92%. Serum PCT median concentrations of gram-negative cultures were higher than those of gram-positive VK groups (p<0.05). PCT can distinguish positive blood cultures sepsis, as well as between different bacterial species.
CONFLICT OF INTERESTS
Non.
REFERENCE
| 1. Le Phan Ngoc Bich (2018), Study of clinical characteristics and serum procalcitonin concentrations in early neonatal infections at Hue University of Medicine and Pharmacy Hospital, Master of Medicine thesis, Hue University of Medicine and Pharmacy. |
| 2. Ministry of Health (2015), Guidelines for the use of antibiotics (Issued together with Decision No. 708/QD-BYT dated March 02, 2015), Hanoi Medical Publishing House, 53. |
| 3. Bui Thi Hong Chau, Le Xuan Truong, Tran Quang Bính et al. (2010), "Value of procalcitonin testing in the diagnosis of sepsis", HCMC Medicine, 14(1), 476–479. |
| 4. Cao Minh Nga (2009), "Bacteremia-causing bacteria and antibiotic resistance", Ho Chi Minh City Medicine, 13(1), 1-7. |
| 5. Tran Thi Thanh Nga, Nguyen Thanh Bao and Cao Minh Nga (2015), "Bacterial agents causing sepsis and antibiotic resistance in the ICU of Cho Ray Hospital", Ho Chi Minh City Medicine, 19(1), 414-420. |
| 6. Nguyen Viet Phuong, Nguyen Minh Hai, Nguyen Van Duong et al. (2017), "Study of the diagnostic value of PCT in patients with sepsis", Journal of Military Medicine and Pharmacology, 6-2017, 79-84. |
| 7. Becker K. L., Snider R., and Nylen E. S. (2008), “Procalcitonin assay in systemic inflammation, infection, and sepsis: clinical utility and limitations”, Critical care medicine, 36(3), 941-952. |
| 8. Bloos F., Marshall J. C., Dellinger R. P. , et al (2011), “Multinational, observational study of procalcitonin in ICU patients with pneumonia requiring mechanical ventilation: a multicenter observational study”, Critical Care, 15(2), 1-9. |
| 9. Davies J. (2015), “Procalcitonin”, Journal of clinical pathology, 68(9), 675-679. |
| 10. JaponiA., Vazin A., Hamedi M., et al. (2009), “Multidrug-resistant bacteria isolated from intensive-care-unit patient samples”, Brazilian Journal of Infectious Diseases, 13(2), 118-122. |
| 11. Kalathia M. B., Shingala P.A., Parmar P.N., et al. (2013), “Study of umbilical cord blood culture in diagnosis of early-onset sepsis among newborns with high-risk factors”, Journal of clinical neonatology, 2(4), 169. |
| 12. Meisner M (2014), “Update on procalcitonin measurements”, Annals of laboratory medicine, 34(4), 263-273. |
| 13. Müller F., Christ-Crain M., Bregenzer T. , et al (2010), “Procalcitonin levels predict bacteremia in patients with community-acquired pneumonia: a prospective cohort trial”, Chest, 138(1), 121-129. |
| 14. Opota O., Croxatto A., Prod'hom G., et al. (2015), “Blood culture-based diagnosis of bacteraemia: state of the art”, Clinical Microbiology and Infection, 21(4), 313-322. |
| 15. Rudd K. E., Johnson S. C., Agesa K. M. , et al (2020), “Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the Global Burden of Disease Study”, The Lancet, 395(10219), 200-211. |
| 16. Self W. H., Balk R. A., Grijalva C. G. , et al (2017), “Procalcitonin as a marker of etiology in adults hospitalized with community-acquired pneumonia”, Clinical Infectious Diseases, 65(2), 183-190. |
| 17. Le Huy Thach, Phan Hung Viet, Le Van Thanh. , et al (2021), “Study clinical, paraclinical features and the outcome of treatment for neonatal infections in early period at Ninh Thuan provincial general hospital ”, Journal Of Functional Ventilation And Pulmonology, 37(12), 26-32. |
| 18. Velaphi S. C., Westercamp M., Moleleki M., et al. (2019), “Surveillance for incidence and etiology of early-onset neonatal sepsis in Soweto, South Africa”, PloS one, 14(4), e0214077. |
| 19. Wacker C., et al (2013), “Procalcitonin as a diagnostic marker for sepsis: a systematic review and meta-analysis”, The Lancet infectious diseases, 13(5), 426-435. |
| 20. Yan S. T., Sun L. C., Jia H. B., et al (2017), “Procalcitonin levels in bloodstream infections caused by different sources and species of bacteria”, American journal of emergency medicine, 35(4), 579-583. |
| 21. Yunus I., Fasih A., and Wang Y. (2018), “The use of procalcitonin in the determination of severity of sepsis, patient outcomes and infection characteristics”, PloS one, 13(1), e020652. |
FIGURE - TABLES
REFERENCE
| 1. Le Phan Ngoc Bich (2018), Study of clinical characteristics and serum procalcitonin concentrations in early neonatal infections at Hue University of Medicine and Pharmacy Hospital, Master of Medicine thesis, Hue University of Medicine and Pharmacy. |
| 2. Ministry of Health (2015), Guidelines for the use of antibiotics (Issued together with Decision No. 708/QD-BYT dated March 02, 2015), Hanoi Medical Publishing House, 53. |
| 3. Bui Thi Hong Chau, Le Xuan Truong, Tran Quang Bính et al. (2010), "Value of procalcitonin testing in the diagnosis of sepsis", HCMC Medicine, 14(1), 476–479. |
| 4. Cao Minh Nga (2009), "Bacteremia-causing bacteria and antibiotic resistance", Ho Chi Minh City Medicine, 13(1), 1-7. |
| 5. Tran Thi Thanh Nga, Nguyen Thanh Bao and Cao Minh Nga (2015), "Bacterial agents causing sepsis and antibiotic resistance in the ICU of Cho Ray Hospital", Ho Chi Minh City Medicine, 19(1), 414-420. |
| 6. Nguyen Viet Phuong, Nguyen Minh Hai, Nguyen Van Duong et al. (2017), "Study of the diagnostic value of PCT in patients with sepsis", Journal of Military Medicine and Pharmacology, 6-2017, 79-84. |
| 7. Becker K. L., Snider R., and Nylen E. S. (2008), “Procalcitonin assay in systemic inflammation, infection, and sepsis: clinical utility and limitations”, Critical care medicine, 36(3), 941-952. |
| 8. Bloos F., Marshall J. C., Dellinger R. P. , et al (2011), “Multinational, observational study of procalcitonin in ICU patients with pneumonia requiring mechanical ventilation: a multicenter observational study”, Critical Care, 15(2), 1-9. |
| 9. Davies J. (2015), “Procalcitonin”, Journal of clinical pathology, 68(9), 675-679. |
| 10. JaponiA., Vazin A., Hamedi M., et al. (2009), “Multidrug-resistant bacteria isolated from intensive-care-unit patient samples”, Brazilian Journal of Infectious Diseases, 13(2), 118-122. |
| 11. Kalathia M. B., Shingala P.A., Parmar P.N., et al. (2013), “Study of umbilical cord blood culture in diagnosis of early-onset sepsis among newborns with high-risk factors”, Journal of clinical neonatology, 2(4), 169. |
| 12. Meisner M (2014), “Update on procalcitonin measurements”, Annals of laboratory medicine, 34(4), 263-273. |
| 13. Müller F., Christ-Crain M., Bregenzer T. , et al (2010), “Procalcitonin levels predict bacteremia in patients with community-acquired pneumonia: a prospective cohort trial”, Chest, 138(1), 121-129. |
| 14. Opota O., Croxatto A., Prod'hom G., et al. (2015), “Blood culture-based diagnosis of bacteraemia: state of the art”, Clinical Microbiology and Infection, 21(4), 313-322. |
| 15. Rudd K. E., Johnson S. C., Agesa K. M. , et al (2020), “Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the Global Burden of Disease Study”, The Lancet, 395(10219), 200-211. |
| 16. Self W. H., Balk R. A., Grijalva C. G. , et al (2017), “Procalcitonin as a marker of etiology in adults hospitalized with community-acquired pneumonia”, Clinical Infectious Diseases, 65(2), 183-190. |
| 17. Le Huy Thach, Phan Hung Viet, Le Van Thanh. , et al (2021), “Study clinical, paraclinical features and the outcome of treatment for neonatal infections in early period at Ninh Thuan provincial general hospital ”, Journal Of Functional Ventilation And Pulmonology, 37(12), 26-32. |
| 18. Velaphi S. C., Westercamp M., Moleleki M., et al. (2019), “Surveillance for incidence and etiology of early-onset neonatal sepsis in Soweto, South Africa”, PloS one, 14(4), e0214077. |
| 19. Wacker C., et al (2013), “Procalcitonin as a diagnostic marker for sepsis: a systematic review and meta-analysis”, The Lancet infectious diseases, 13(5), 426-435. |
| 20. Yan S. T., Sun L. C., Jia H. B., et al (2017), “Procalcitonin levels in bloodstream infections caused by different sources and species of bacteria”, American journal of emergency medicine, 35(4), 579-583. |
| 21. Yunus I., Fasih A., and Wang Y. (2018), “The use of procalcitonin in the determination of severity of sepsis, patient outcomes and infection characteristics”, PloS one, 13(1), e020652. |
ARTICLE INFO DOI: 10.12699/jfvpulm.13.40.2022.1
Conflict of Interest
Non
Date of manuscript receiving
18/01/2022
Date of publication after correction
18/04/2022
Article citation
Le Huy T, Le Van T, Do Thuy D, Le Quoc T. Serum Procalcitonin Levels And Association With Blood Culture Results At General Hospital Ninh Thuan Province In 2021. J Func Vent Pulm 2022;40(12):1-6