
 English
English
 French
French
Discriminative threshold value and diagnostic performance of adenosine deaminase in tuberculous serofibrinous pleurisy
Valeur seuil discriminante et performance diagnostique de l' adénosine désaminase dans la pleurésie sérofibrineuse tuberculeuse
Paulvon Phérol Koumeka1,2, Presley Lee Bemba2,3, Glad Smart Moussounda Mpika1, Lamyae Amro1
1: Department of Respiratory Medicine, Arrazi Hospital, Mohammed VI University Hospital Center’s, LRMS, Faculty of Medicine and Pharmacy, Cadi Ayyad University, Marrakech, Morocco
2: Brazzaville University Hospital Center’s, Pneumology Department, Brazzaville, Congo
3: Marien N’GOUABI University, Brazzaville, Congo
Corresponding author: PAULVON PHEROL KOUMEKA. Brazzaville University Hospital Center’s Pneumology Department, Brazzaville, Congo
E-mail: paulvonpherolkoumeka@gmail.com
ABSTRACT
Introduction. Pleural adenosine deaminase (ADA) is a useful diagnosis test for the tuberculous pleurisy, but its exact cutoff of positivity and its accuracy in clinical decision making are unclear. Objective. To determine the threshold and diagnostic performance of pleural ADA in tuberculous serum pleurisy. Methods: This is a diagnostic, case-control study including patients admitted to day hospitals for serous pleurisy. Cases were defined by histopathology suggestive of tuberculosis and/or by identification of BK and/or its genome by geneXpert, by culture on pleural fluid and/or on pleural biopsy specimen. Results: 209 patients were included. The AUC of the ROC curve of ADA was 0.75. The ADA threshold was 49 IU/L, with a sensitivity of 60.6%, specificity of 78.3%, positive predictive value (PPV) of 85.1%, positive likelihood ratio (PLR) of 2.8, odds ratio (OR) of 5.5 [2.5; 12.5], kappa agreement at 0.34. For ADA<36 IU/L, the PPV, PLR, OR were 36.4%, 0.3 and 0.17 [0.1; 0.4], respectively; with p=0.0000. The two groups with ADA thresholds 36 to 44, 45 to 65 IU/L, gave similar PLR close to one. For ADA> 65 IU/L, the PPV, sensitivity, specificity, PLR, OR were 89.2%, 35.1, 91.3%, 4 and 5.7 [1.9; 17.2], respectively; with p=0.0000. Conclusion. Our study shows that pleural ADA is a moderately discriminating test for the diagnosis of tuberculous serum pleurisy. The ADA cutoff was 49 IU/L. Two ADA cutoffs are useful in the diagnosis of serofibrinous pleurisy. ADA values<36 IU/L exclude the diagnosis and those >65 IU/L are suggestive of pleural tuberculosis.
KEYWORDS: Adenosine deaminase; Serofibrinous pleurisy; Tuberculosis.
RÉSUMÉ
Introduction L'adénosine désaminase pleurale (ADA) est un test de diagnostic utile pour la pleurésie tuberculeuse, mais son seuil exact de positivité et son exactitude dans la prise de décision clinique ne sont pas clairs. Objectif. Déterminer le seuil et les performances diagnostiques de l’ADA pleurale dans la pleurésie sérique tuberculeuse. Méthodes. Il s'agit d'une étude diagnostique cas-témoins incluant des patients admis dans des hôpitaux de jour pour pleurésie séreuse. Les cas ont été définis par une histopathologie évocatrice de tuberculose et/ou par identification de BK et/ou de son génome par geneXpert, par culture sur liquide pleural et/ou sur biopsie pleurale. Résultats : 209 patients ont été inclus. L'AUC de la courbe ROC de l'ADA était de 0,75. Le seuil ADA était de 49 UI/L, avec une sensibilité de 60,6 %, une spécificité de 78,3 %, une valeur prédictive positive (VPP) de 85,1 %, un rapport de vraisemblance positif (PLR) de 2,8, un odds ratio (OR) de 5,5 [2,5 ; 12,5], accord kappa à 0,34. Pour l’ADA < 36 UI/L, la VPP, le PLR et l’OR étaient de 36,4 %, 0,3 et 0,17 [0,1 ; 0,4], respectivement ; avec p=0,0000. Les deux groupes avec des seuils ADA de 36 à 44, 45 à 65 UI/L, ont donné un PLR similaire, proche de un. Pour ADA > 65 UI/L, la VPP, la sensibilité, la spécificité, le PLR, l'OR étaient de 89,2 %, 35,1, 91,3 %, 4 et 5,7 [1,9 ; 17.2], respectivement ; avec p=0,0000. Conclusion. Notre étude montre que l’ADA pleurale est un test moyennement discriminant pour le diagnostic de pleurésie sérique tuberculeuse. Le seuil de l’ADA était de 49 UI/L. Deux seuils ADA sont utiles dans le diagnostic de la pleurésie sérofibrineuse. Les valeurs d'ADA < 36 UI/L excluent le diagnostic et celles > 65 UI/L sont évocatrices d'une tuberculose pleurale.
MOTS CLÉS: Adénosine désaminase; Pleurésie sérofibrineuse; Tuberculose.
INTRODUCTION
Globally, it is estimated that approximately 10 million people contracted tuberculosis (TB) in 2019. TB is one of the top 10 causes of death and the leading cause of death from an infectious agent [1]. The disease usually affects the lungs but can also have other locations (extra pulmonary TB), including the pleura. Morocco remains a tuberculosis endemic country with an incidence of extra pulmonary localizations of tuberculosis increasing from 23 to 46%, between 1980 and 2015, dominated by lymph node and pleural localization [2].
The definitive diagnosis of pleural tuberculosis can only be confirmed by identification of the Mycobacterium tuberculosis (MT), either by microscopy and/or cultures from sputum, pleural fluid and pleural biopsy specimens.
Unfortunately, these procedures are only accomplished in few cases. In our recently published series, culture and geneXpert in pleural fluid had a sensitivity of 3 and 42%, respectively [3]. The low sensitivity of these microbiological investigations is partly explained by the low incidence of HIV-tuberculosis comorbidity. In Morocco, the burden of HIV-related TB is estimated to be 1.8% [1]. In HIV-infected individuals, microbiological investigations are more sensitive, probably due to impaired bacterial clearance from the pleural space in the context of immunosuppression.
The low yield of microbiologic studies and the invasiveness of pleural biopsy have stimulated the search for biomarkers in tuberculous pleural fluid. Although many have been evaluated in recent decades, the most qualified is pleural adenosine deaminase. Previous meta-analyses have highlighted a good diagnostic performance of pleural ADA in the diagnosis of pleural TB [4, 5, 6, 7]. Indeed, pleural ADA is a useful biomarker in the diagnosis of pleural TB. In countries with a high incidence of TB, high ADA values are associated with pleural TB [4, 8]. Pleural ADA assay may supplant pleural biopsy in the setting of TB endemicity.
Despite strong evidence, almost four decades later, there is still some controversy about the place of pleural ADA in expedited decision-making in the diagnosis of TB [4]. Moreover, there is still no clarity on an optimal discriminative cutoff value. Our recently published study and previous meta-analyses have not addressed this threshold issue in detail [3, 4]. Only one meta-analysis to our knowledge has addressed this ADA threshold issue[4]. However, this meta-analysis, as the authors point out, has methodological limitations. Most of the included articles were not based on microbiological and/or pathological criteria to confirm the tuberculous origin of the pleural effusion [4]. This study was conducted to determine the optimal discriminative cutoff value and diagnostic performance of pleural ADA in the diagnosis of tuberculous serum pleurisy.
METHODS
Study design and setting
This was a diagnostic, case-control study with retrospective data collection, conducted from January 01, 2015 to August 30, 2020, at the Mohammed VI University Hospital of Marrakech, in the department of pneumology.
Participants
The study interested patients admitted in day hospital for serofibrinous pleurisy. Patients aged 18 years and older with lymphocytic exudative pleurisy were included, those who had undergone a blind pleural biopsy. Patients with superinfected serofibrinous pleurisy, purulent pleurisy and those with histopathological examination of a granulomatous lesion without necrosis leaving the diagnosis of tuberculosis in doubt, were excluded from the study.
The study sample, consisting of patients matching the inclusion criteria, was obtained by simple random selection. The minimum expected sample size for a representative population sample was calculated from the Schwarz formula [9]:
n = z2 x p x (1-p)/α2
n: minimum sample size representative of the study population; z: 95% confidence level = 1.96; α: margin of error set at 5%; p: the prevalence of the disease.
The minimum sample size calculated for representative population was 138 patients.
Two study groups were distinguished:
Cases: patients in whom the tuberculous origin of the pleurisy was confirmed by histopathology and/or by identification of BK and/or its genome by geneXpert and/or by culture on pleural fluid and/or by culture on pleural biopsy fragment.
Controls: patients with serofibrinous pleurisy of etiology other than tuberculosis.
Variables
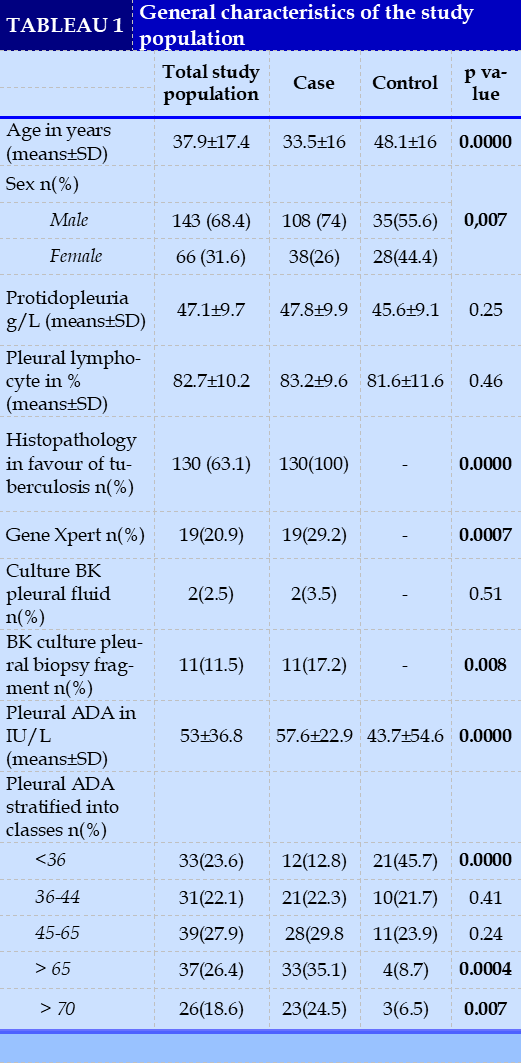
The study variables, socio-demographic, clinical, paraclinical, were defined. The pleural ADA variable (in IU/L) was stratified into 5 cutoff categories: <36, 36-44, 45-65, >65, and >70.
The pleural ADA cutoff was determined from receiver operating function analysis (ROC curve).
The diagnostic performance of pleural ADA was studied by calculating the predictive values of pleural ADA.
Criteria of judgement
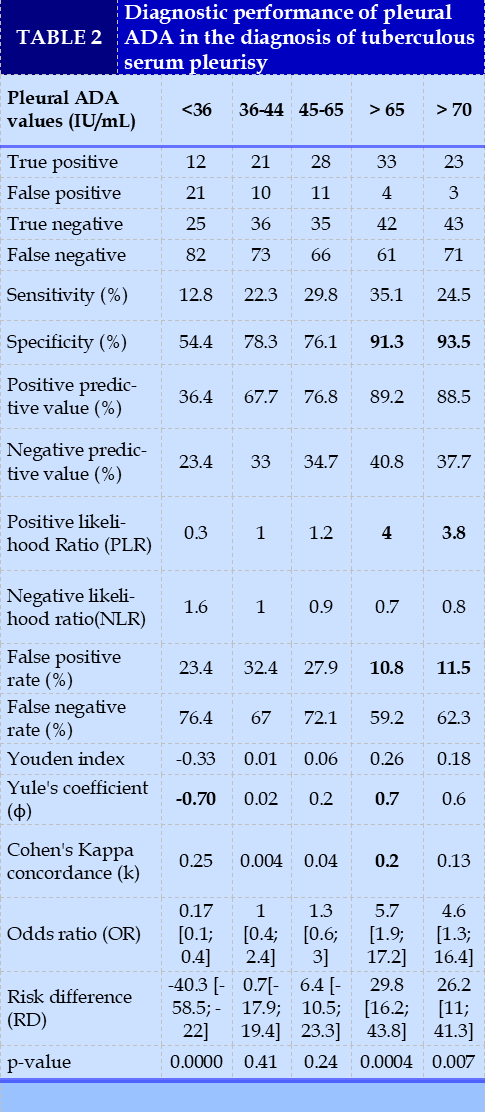
The criteria for evaluating the diagnostic performance of pleural ADA were: sensitivity (Se), specificity (Sp), positive predictive value (PPV), negative predictive value (NPV), positive likelihood ratio (PLR) and negative (NLR), Youden's index, Yule's coefficient, Cohen's kappa concordance (k), and area under the ROC curve (AUC) of pleural ADA.
Thus:
Youden index equal to 0 test zero, negative value, the test is said to be ineffective; when the test approaches 1, it is said to be effective.
Yule's coefficient equal to 0, zero test, negative value, the test is said to be ineffective; when the test is close to 1, it is said to be effective.
AUC = 0.5, null contribution test, 0.5 ≤ AUC < 0.7, weakly discriminating test 0.7 ≤ AUC < 0.9, moderately discriminating test, 0.9 ≤ AUC < 1, highly discriminating test, and AUC = 1, perfect test.
Positive likelihood ratio (PLR): > 10, 5-10, 2-5, 1-2 and 1, correspond to very high, high, moderate, low and no diagnostic input, respectively.
Negative likelihood ratio (NLR): < 0.1, 0.1-0.2, 0.2-0.5, 0.5-1, 1, correspond to very high, high, moderate, low and no diagnostic input, respectively.
Cohen's kappa agreement: k< 0, disagreement of the two tests; 0.0 <k< 0.20 very weak agreement, 0.21 < k<0.40 weak agreement, 0.41<k<0.60 moderate agreement, 0.61 <k< 0.80 strong agreement, and 0.80 <k< 1.00 almost perfect agreement.
Data sources
Data collection was done using the computerized electronic data recording system.
Statistical Analysis
Data were entered into Excel 2016 and analyzed on Epi info version 7.2.2.16. Categorical variables were expressed as headcount and percentage, quantitative variables as mean and standard deviation. The Mid-p exact and Fisher exact tests (depending on the number) were used for the comparison of qualitatives variables and the Mann-Whitney/Wilcoxon test (Kruskal-Wallis test) for the comparison of quantitative variables.
The diagnostic performance of the test was studied by calculating the sensitivity (Se), specificity (Sp), positive (PLR) and negative (NLR) likelihood ratios, positive predictive value (PPV), negative predictive value (NPV).
The effectiveness of the test was determined by calculation of the Youden index, and by the receiver operating characteristic (ROC), as well as by the PLR and NLR.
The reliability of the test was studied by calculating the concordance coefficient Kappa (k).
The ADA cutoff was determined from the ROC curve.
For the tests of data comparisons, the threshold of significance retained was the value of p <0.05.
Patient with missing data, including pleural ADA value, were not included in the calcul of pleural ADA predictives values.
RESULTS
A total of 209 patients were included. Tuberculosis was diagnosed in 69.9% (n=146) of cases and was the most frequent etiology of serous pleurisy. Figure 1 shows the general distribution of the study population according to study group.
The average age of the included patients was 37.9 ± 17.4 years (18 to 85 years). Male gender was predominant with a sex of 2.2.
For 67% (n=140) of the patients the pleural ADA was measured. The pleural ADA value averaged 57.6±22.8 versus 43.7±54.6 in controls, with a statistically significant difference (p value<0.0001). The area under the ROC curve of pleural ADA was 0.75.
ROC curve analysis determined the pleural ADA cutoff at 49 IU/L, with a sensitivity of 60.6%, specificity of 78.3%, positive predictive value (PPV) of 85.1%, negative predictive value of 49. 3%, positive likelihood ratio (PLR) of 2.8, negative likelihood ratio (NLR) of 0.5, odds ratio (OR) of 5.5 [2.5; 12.5], Youden index of 0.4 kappa agreement of 0.34 and p<0.0001.
The data set of general patient characteristics as well as the diagnostic performance of pleural ADA are represented in Tables 1 and 2, respectively.
DISCUSSION
We included 209 patients. The study sample size was representative of the general population. From a methodological point of view, our study had the advantage that the included patients had received a blind pleural biopsy. Furthermore, cases were defined on the basis of microbiological and pathological evidence, which provided precision in the diagnosis of tuberculous serofibrinous pleurisy.
The pleural ADA variable was stratified into 5
threshold categories. The same threshold categories are reported in the 2 meta-analyses by Aggarwal AN et al [4,10]. These thresholds were chosen on the basis of general expert opinions that ADA levels below 40 IU/L can exclude a diagnosis of tuberculous pleural effusion [4,5]. In our study we added the threshold of pleural ADA > 70 IU/L because there are few studies evaluating this threshold [4].
Pleural tuberculosis was the most frequent etiology of serous tuberculous pleurisy. In fact, tuberculosis is the leading cause of lymphocytic exudative pleurisy in tuberculosis endemic countries [3, 11, 12].
The mean ADA value was higher in cases compared to controls with a statistically significant difference. Indeed, high pleural ADA values are associated with the tuberculous origin of pleurisy.
The overall analysis of the predictive values, showed an increase in overall performance of the test with increasing defined thresholds of positivity (Table 2), contrary to the data of the meta-analysis of Aggarwal AN et al which reported similar values of sensitivity and specificity for the 4 categories of thresholds of ADA <36, 36 to 44, 45 to 65, > 65 IU/L [4]. This difference in results may be explained by the quality of the studies included in the meta-analysis by Aggarwal AN et al. Many studies included patients with transudative effusion, which may have improved the specificity estimates. Similarly, very few studies included only lymphocytic exudative effusions, which may have improved the sensitivity of the test; tuberculosis is the most common etiology of lymphocytic exudative pleurisy.
Receiver operating characteristic (ROC) curve analysis showed that pleural ADA is a moderately discriminating test with AUC at 0.75. The cutoff value determined from the ROC curve was 49 IU/L. For a cutoff value at 49 IU/L, pleural ADA with a high positive predictive value 85.1%, an odds ratio of 5.5 [2.5; 12.5]. This threshold was associated with the tuberculous origin of the serous pleurisy (p<0.0001).
Our study corroborates the data in the literature. In fact, four meta-analyses underline the high diagnostic performance of ADA for a cutoff of 40 IU/L [6, 10, 13, 14]. Pleural ADA values below 36 IU/L were very significantly associated with non-tuberculous origin of pleurisy. In fact, based on general expert opinion, ADA levels below 40 IU/L have an excellent negative predictive value for excluding the diagnosis of tuberculosis [4, 5, 15-17].
The two groups with ADA thresholds 36 to 44 and 45 to 65 IU/L, gave similar positive (PLR) and negative (NLR) likelihood ratios close to one (1) and non-significant odds ratios (Table 2). These ADA thresholds were uninformative. Therefore, for ADA thresholds 36 to 44 and 45 to 65 IU/L, a search for a differential diagnosis is required. Patients should require further investigation to substantiate the etiology of serum pleurisy. Pleural biopsy may be useful in this setting [4, 19]. Indeed, high levels of ADA in serofibrinous pleurisy may be associated with other neoplastic causes such as lymphomas, solid tumors [8, 18, 19], and connective tissue deseases [18, 20, 21]. In our study, neoplastic causes of serofibrinous pleurisy accounted for 10.5% (6.7; 15.5%) and connective tissue deseases 0.5% (0.01; 2.6%).
For ADA thresholds > 65 IU/L and > 70 IU/L, the positive predictive value, specificity, and PLR ratio were high, with a statistically significant odds ratio and confidence interval. We deduce that a high level of ADA (> 65 IU/L) may be more suggestive of tuberculous serum pleurisy. The same observation is reported by the meta-analysis of Aggarwal AN et al who concluded that empirical anti-tuberculosis treatment can be considered in these patients [4].
CONCLUSION
Our study shows that pleural ADA is a moderately discriminating test for the diagnosis of tuberculous serous pleurisy. The ADA cutoff was 49 IU/L, however for ADA values between 36 and 65 IU/L patients should require further investigations such as pleural biopsy. Therefore, two ADA thresholds are useful for the diagnosis of serofibrinous pleurisy; ADA values < 36 IU/L exclude the diagnosis and those >65 IU/L are suggestive of pleural tuberculosis.
CONFLICT OF INTERESTS
Non.
REFERENCE
| 1. World health organization. (2020). Global tuberculosis report 2020: executive summary 2020. World health organization. https://apps.who.int/iris/handle/10665/337538. License: CC BY-NC-SA 3.0 IGO. |
| 2. National Tuberculosis Control Program. National strategic plan for the prevention and control of tuberculosis in Morocco 2018-2021. https://www.smmg.ma/publications/documents/1-programme-national-de-lutte-contre-tuberculose. |
| 3. Kouméka PP, Ouldittou I, Fikri O, Saidi I, Ait Batahar S, Amro L. Predictive factors and biomarkers of tuberculous lymphocytic exudative pleurisy. Rev Mal Respir. 2021 Mar;38(3):231-239. https://doi.org/10.1016/j.rmr.2020.11.001. |
| 4. Aggarwal AN, Agarwal R, Sehgal IS, Dhooria S. Adenosine deaminase for diagnosis of tuberculous pleural effusion: A systematic review and meta-analysis. PLoS One. 2019 Mar 26;14(3):e0213728. doi: 10.1371/journal.pone.0213728. PMID: 30913213; PMCID: PMC6435228. |
| 5. Mollo B, Jouveshomme S, Philippart F, Pilmis B. Biological markers in the diagnosis of tuberculous pleural effusion. Ann Biol Clin (Paris). 2017 Feb 1;75(1):19-27. English. doi: 10.1684/abc.2016.1201. PMID: 28057604. |
| 6. Goto M, Noguchi Y, Koyama H, Hira K, Shimbo T, Fukui T. Diagnostic value of adenosine deaminase in tuberculous pleural effusion: a meta-analysis. Ann Clin Biochem. 2003;40(Pt 4): 374-81. |
| 7. Palma RM, Bielsa S, Esquerda A, Martínez-Alonso M, Porcel JM. Diagnostic Accuracy of Pleural Fluid Adenosine Deaminase for Diagnosing Tuberculosis. Meta-analysis of Spanish Studies. Arch Bronconeumol (Engl Ed). 2019 Jan;55(1):23-30. English, Spanish. doi: 10.1016/j.arbres.2018.05.007. Epub 2018 Jun 30. PMID: 30612601. |
| 8. Lee YC, Rogers JT, Rodriguez RM, Miller KD, Light RW. Adenosine deaminase levels in nontuberculous lymphocytic pleural effusions. Chest. 2001 Aug;120(2):356-61. doi: 10.1378/chest.120.2.356. PMID: 11502629. |
| 9. Schwartz D. Statistical methods for physicians and biologists. Ed. Flammarion Medecins Sciences; 1964. P.1004; https://www.persee.fr/doc/pop_0032- 4663 1964_ num_ 19_5_11377. |
| 10. Aggarwal AN, Agarwal R, Sehgal IS, Dhooria S, Behera D. Meta-analysis of indian studies evaluating adenosine deaminase for diagnosing tuberculous pleural effusion. Int J Tuberc Lung Dis.2016; 20(10):1386-91. https://doi.org/10.5588/ijtld.16.0298 |
| 11. Sharma SK, Mohan A. Extrapulmonary tuberculosis. Indian J Med Res. 2004 Oct;120(4):316-53. PMID: 15520485 |
| 12. Batungwanayo J, Taelman H, Allen S, Bogaerts J, Kagame A, Van de Perre P. Pleural effusion, tuberculosis and HIV-1 infection in Kigali, Rwanda. AIDS. 1993 Jan;7(1):73-9. Greco S, Girardi E, Masciangelo R, Capoccetta GB, Saltini C. Adenosine deaminase and interferon gamma measurements for the diagnosis of tuberculous pleurisy: a meta-analysis. Int J Tuberc Lung Dis. 2003 Aug;7(8):777-86. PMID: 12921155. |
| 13. Liang QL, Shi HZ, Wang K, Qin SM, Qin XJ. Diagnostic accuracy of adenosine deaminase in tuberculous pleurisy: a meta-analysis. Respir Med. 2008 May;102(5):744-54. |
| 15. Leila Antonangelo, Caroline S. Faria & Roberta K. Sales (2019): Tuberculous pleural effusion: diagnosis & management, Expert Review of Respiratory Medicine, DOI: 10.1080/17476348.2019.1637737 |
| 16. Light RW. Update on tuberculous pleural effusion. Respirology. 2010 Apr;15(3):451-8. doi: 10.1111/j.1440-1843.2010.01723.x. Epub 2010 Mar 21. PMID: 20345583. |
| 17. Gopi A, Madhavan SM, Sharma SK, Sahn SA. Diagnosis and treatment of tuberculous pleural effusion in 2006. Chest. 2007 Mar;131(3):880-889. doi: 10.1378/chest.06-2063. PMID: 17356108. |
| 18. Maturu VN, Dhooria S, Bal A, Singh, Aggarwal AN, Gupta D, et al. Role of medical thorascopy and closed-blind pleural biopsyin undiagnosed exudative pleural effusion: a single-center experience of 384 patients. J Bronchology Interv Pulmonol. 2015; 22(2):121-9. https://doi.org/10.1097/LBR.0000000000000145. |
| 19. Antonangelo L, Vargas FS, Genofre EH, Oliveira CM, Teixeira LR, Sales RK. Differentiating between tuberculosis-related and lymphoma-related lymphocytic pleural effusions by measuring clinical and laboratory variables: is it possible? J Bras Pneumol. 2012 Mar-Apr;38(2):181-7. English, Portuguese. doi: 10.1590/s1806-37132012000200006. PMID: 22576425. |
| 20. Wong PC. Management of tuberculous pleuritis: can we do better? Respirology. 2005 Mar;10(2):144-8. doi: 10.1111/j.1440-1843.2005.00689.x. PMID: 15823177. |
| 21. Skouras VS, Kalomenidis I. Pleural fluid tests to diagnose tuberculous pleuritis. Curr Opin Pulm Med. 2016 Jul;22(4):367-77. doi: 10.1097/MCP.0000000000000277. PMID: 27064428. |
Figures - Tables
REFERENCE
| 1. World health organization. (2020). Global tuberculosis report 2020: executive summary 2020. World health organization. https://apps.who.int/iris/handle/10665/337538. License: CC BY-NC-SA 3.0 IGO. |
| 2. National Tuberculosis Control Program. National strategic plan for the prevention and control of tuberculosis in Morocco 2018-2021. https://www.smmg.ma/publications/documents/1-programme-national-de-lutte-contre-tuberculose. |
| 3. Kouméka PP, Ouldittou I, Fikri O, Saidi I, Ait Batahar S, Amro L. Predictive factors and biomarkers of tuberculous lymphocytic exudative pleurisy. Rev Mal Respir. 2021 Mar;38(3):231-239. https://doi.org/10.1016/j.rmr.2020.11.001. |
| 4. Aggarwal AN, Agarwal R, Sehgal IS, Dhooria S. Adenosine deaminase for diagnosis of tuberculous pleural effusion: A systematic review and meta-analysis. PLoS One. 2019 Mar 26;14(3):e0213728. doi: 10.1371/journal.pone.0213728. PMID: 30913213; PMCID: PMC6435228. |
| 5. Mollo B, Jouveshomme S, Philippart F, Pilmis B. Biological markers in the diagnosis of tuberculous pleural effusion. Ann Biol Clin (Paris). 2017 Feb 1;75(1):19-27. English. doi: 10.1684/abc.2016.1201. PMID: 28057604. |
| 6. Goto M, Noguchi Y, Koyama H, Hira K, Shimbo T, Fukui T. Diagnostic value of adenosine deaminase in tuberculous pleural effusion: a meta-analysis. Ann Clin Biochem. 2003;40(Pt 4): 374-81. |
| 7. Palma RM, Bielsa S, Esquerda A, Martínez-Alonso M, Porcel JM. Diagnostic Accuracy of Pleural Fluid Adenosine Deaminase for Diagnosing Tuberculosis. Meta-analysis of Spanish Studies. Arch Bronconeumol (Engl Ed). 2019 Jan;55(1):23-30. English, Spanish. doi: 10.1016/j.arbres.2018.05.007. Epub 2018 Jun 30. PMID: 30612601. |
| 8. Lee YC, Rogers JT, Rodriguez RM, Miller KD, Light RW. Adenosine deaminase levels in nontuberculous lymphocytic pleural effusions. Chest. 2001 Aug;120(2):356-61. doi: 10.1378/chest.120.2.356. PMID: 11502629. |
| 9. Schwartz D. Statistical methods for physicians and biologists. Ed. Flammarion Medecins Sciences; 1964. P.1004; https://www.persee.fr/doc/pop_0032- 4663 1964_ num_ 19_5_11377. |
| 10. Aggarwal AN, Agarwal R, Sehgal IS, Dhooria S, Behera D. Meta-analysis of indian studies evaluating adenosine deaminase for diagnosing tuberculous pleural effusion. Int J Tuberc Lung Dis.2016; 20(10):1386-91. https://doi.org/10.5588/ijtld.16.0298 |
| 11. Sharma SK, Mohan A. Extrapulmonary tuberculosis. Indian J Med Res. 2004 Oct;120(4):316-53. PMID: 15520485 |
| 12. Batungwanayo J, Taelman H, Allen S, Bogaerts J, Kagame A, Van de Perre P. Pleural effusion, tuberculosis and HIV-1 infection in Kigali, Rwanda. AIDS. 1993 Jan;7(1):73-9. Greco S, Girardi E, Masciangelo R, Capoccetta GB, Saltini C. Adenosine deaminase and interferon gamma measurements for the diagnosis of tuberculous pleurisy: a meta-analysis. Int J Tuberc Lung Dis. 2003 Aug;7(8):777-86. PMID: 12921155. |
| 13. Liang QL, Shi HZ, Wang K, Qin SM, Qin XJ. Diagnostic accuracy of adenosine deaminase in tuberculous pleurisy: a meta-analysis. Respir Med. 2008 May;102(5):744-54. |
| 15. Leila Antonangelo, Caroline S. Faria & Roberta K. Sales (2019): Tuberculous pleural effusion: diagnosis & management, Expert Review of Respiratory Medicine, DOI: 10.1080/17476348.2019.1637737 |
| 16. Light RW. Update on tuberculous pleural effusion. Respirology. 2010 Apr;15(3):451-8. doi: 10.1111/j.1440-1843.2010.01723.x. Epub 2010 Mar 21. PMID: 20345583. |
| 17. Gopi A, Madhavan SM, Sharma SK, Sahn SA. Diagnosis and treatment of tuberculous pleural effusion in 2006. Chest. 2007 Mar;131(3):880-889. doi: 10.1378/chest.06-2063. PMID: 17356108. |
| 18. Maturu VN, Dhooria S, Bal A, Singh, Aggarwal AN, Gupta D, et al. Role of medical thorascopy and closed-blind pleural biopsyin undiagnosed exudative pleural effusion: a single-center experience of 384 patients. J Bronchology Interv Pulmonol. 2015; 22(2):121-9. https://doi.org/10.1097/LBR.0000000000000145. |
| 19. Antonangelo L, Vargas FS, Genofre EH, Oliveira CM, Teixeira LR, Sales RK. Differentiating between tuberculosis-related and lymphoma-related lymphocytic pleural effusions by measuring clinical and laboratory variables: is it possible? J Bras Pneumol. 2012 Mar-Apr;38(2):181-7. English, Portuguese. doi: 10.1590/s1806-37132012000200006. PMID: 22576425. |
| 20. Wong PC. Management of tuberculous pleuritis: can we do better? Respirology. 2005 Mar;10(2):144-8. doi: 10.1111/j.1440-1843.2005.00689.x. PMID: 15823177. |
| 21. Skouras VS, Kalomenidis I. Pleural fluid tests to diagnose tuberculous pleuritis. Curr Opin Pulm Med. 2016 Jul;22(4):367-77. doi: 10.1097/MCP.0000000000000277. PMID: 27064428. |
ARTICLE INFO DOI: 10.12699/jfvpulm.14.44.2023.27
Conflict of Interest
Non
Date of manuscript receiving
25/04/2023
Date of publication after correction
25/10/2023
Article citation
Paulvon Phérol Koumeka, Presley Lee Bemba, Glad Smart Moussounda Mpika, Lamyae Amro. J Func Vent Pulm 2023;44(14):27-32