
 English
English
 French
French
Ischemic stroke in a patient with tuberculous meningitis and HIV infection
AVC ischémique chez un patient atteint de méningite tuberculeuse et d'infection par le VIH
Mohamed Amine Mnaili1,2, Ahmed Bourazza3
1: Neurology departement, Agadir Military Hospital, Agadir, Morocco
2: University of Hassan II, Casablanca, Morocco
3: Neurology departement, Mohammed V Military Hospital, Rabat, Morocco
Corresponding author:
MNAILI Mohamed Amine. Neurology departement, Agadir Military Hospital, Agadir, Morocco.
E-mail: aminemed08@gmail.com
ABSTRACT
Tuberculous meningitis (TBM) is a devastating disease. TBM occurs more commonly in HIV infected patients. The influence of HIV co-infection on clinical manifestations and outcome of TBM is not well defined. Yet, some differences have been observed and stroke has been recorded to occur more frequently. This case reports on an HIV infected female with lung, meningeal tuberculosis and stroke due to a cortical sub-cortical ischemic lesion. This case argues that tuberculosis must be considered as the etiology of a stroke especially during HIV infection.
Miliary tuberculosis is a hematogenous dissemination of Mycobacterium Tuberculosis which involves especially lungs, central nervous system and lymph node. An acute cerebrovascular event may occur in the course of an underlying disease, and the infection may be the etiological cause of cerebral infarction when associated with other underlying infectious conditions.TBM must be included in the differential diagnosis of HIV infected patients with stroke and TBM treatment needs be started as soon as possible before the onset of vasculopathy.
KEYWORDS: Tuberculosis; Miliary; Meningitis; Acute ischemic stroke; HIV infection.
RÉSUMÉ
La méningite tuberculeuse (TBM) est une maladie dévastatrice. La TBM survient plus fréquemment chez les patients infectés par le VIH. L'influence de la co-infection par le VIH sur les manifestations cliniques et l'issue du TBM n'est pas bien définie. Pourtant, certaines différences ont été observées et les accidents vasculaires cérébraux sont plus fréquents. Nous rapportons le cas d’une patiente infectée par le VIH et présentant d'une tuberculose pulmonaire et méningée compliqué d’un accident vasculaire cérébral ischémique corticale sous-corticale. Ce cas argumente que la tuberculose doit être évoquée comme étiologie d’un AVCI, surtout lors d’une infection au VIH.
La tuberculose miliaire est une dissémination hématogène de Mycobacterium Tuberculosis qui touche notamment les poumons, le système nerveux central et les ganglions lymphatiques. Un événement vasculaire cérébral aigu peut survenir au cours d'une maladie sous-jacente et l'infection peut être la cause étiologique d'un infarctus cérébral lorsqu'elle est associée à d'autres affections infectieuses sous-jacentes. Le TBM doit être inclus dans le diagnostic différentiel des patients infectés par le VIH ayant subi un accident vasculaire cérébral et un traitement par TBM. doit être débuté le plus tôt possible avant l’apparition de la vasculopathie.
MOTS CLÉS: Tuberculose; Miliaire; Méningite; AVC ischémique aigu; HIV infection.
INTRODUCTION
Tuberculosis (TB) is a pressing global health challenge with profound implications for public health, society, and economies [1] According to World Health Organization (WHO) data, tuberculosis represents one of the top 10 global causes of mortality. In 2016, 10.4 million people became ill with tuberculosis and 1.7 million died because of it [2].
Tuberculosis is often thought of as an infectious disease with progressive pulmonary involvement (pulmonary tuberculosis), it can possibly affect any organ system (extrapulmonary tuberculosis).
Extrapulmonary tuberculosis at the level of the central nervous system is the most devastating and deadly form of tuberculosis. This presentation represents approximately 1% of the total cases of tuberculosis and 6–10% of the extrapulmonary forms in immunocompetent patients [3]. For this reason, tuberculosis at the brain level represents a significant challenge at the time of diagnosis.
Meningitis (TBM) is the most frequent presentation of CNS TB, with its most serious consequence being brain infarction [4]. Most TBM associated brain infarcts are multiple, bilateral, symmetric, located in the basal ganglia, anterior thalamus, anterior limb and the genu of internal capsule [5,6]. Cortical, sub-cortical white matter, brainstem and hindbrain involvement are less common, except in cases prolonged by TB treatment and HIV co-infection [5,7].
Interestingly, ischemic stroke has been reported more frequently in a cohort of patients with a diagnosis of TB not involving CNS over 3 years of follow up, indicating that TB and not only TBM is a potential risk factor for stroke [8]. Our case report describes a HIV co-infected female who developed stroke due to TBM secondary vasculopathy with brain infarction of the anterior cerebral artery.
CASE REPORT
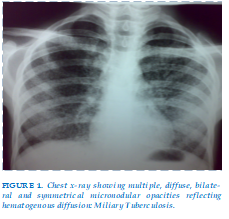
A 45-year-old women with HIV infection was admitted to a hospital complaining of fever and cough and dyspnea which began a week prior. On admission: patient was conscious, polypnea FR 26 cycles/min, tachycardic 125 pbm , SaO2 88%. Pulmonary auscultation found bilateral crackling sounds. The erythrocyte sedimentation rate (ESR) 75 mm 1st hour, hemoglobin 11,5 white blood cells 5500 mm3, neutrophils 75 % lymphocytes 19 % coagulation liver and renal functional tests were normal. A chest radiograph depicted micronodular shadowing consistent with miliary TB. (Figure 1) A bronchoscopy did not show lesions. Bronco-alveolar lavage (BAL) fluid was negative for acid fast bacilli (AFB) and M. tuberculosis complex strand displacement amplification (SDA) was non-reactive. Culture subsequently identified multisusceptible Mycobacterium tuberculosis.
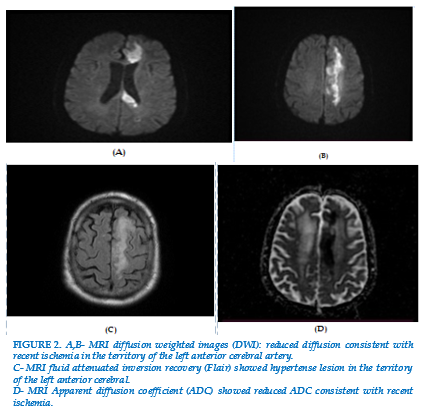
On the same day, in the absence of neurologic symptoms, cerebrospinal fluid (CSF) was examined. Analysis of cerebrospinal fluid showed: glucose CSF 0,25 g/dL dl (blood glucose 0,93 g/dL), white cells 150/mm3 and proteins 3,51g/dL. GeneXpert was positive in sputum and cerebrospinal fluid. The diagnosis of miliary tuberculosis with meningitis was established. Antituberculosis treatment Rifampicin 600 mg/ day, Isoniazid 300 mg/day, ethambutol 1200 mg/ day and pyrazinamide 1500 mg/day was started. It was associated with corticosteroids (dexamethasone 4 mg) and oxygen. Six days later, the patient presents hemiparesis in right side, weakness and dysarthria.. A brain CT depicted a left frontal cortical sub-cortical post ischemic high density lesion. Magnetic resonance (MRI) and angio-MRI (Figure 2) also depicted recent ischemic cortical sub-cortical lesion without hemorrhagic transformation, absence of flow through the left anterior cerebral artery. Thus, the patient was diagnosed with TBM complicated with stroke.
DISCUSSION
Miliary tuberculosis is a hematogenous dissemination of Mycobacterium Tuberculosis which involves especially lungs, central nervous system and lymph node.
Among the different presentations of disseminated tuberculosis, meningeal tuberculosis is the most infrequent clinical form. Particular clinical features are not characteristic, making it extremely difficult to identify and complete a timely diagnosis. Such difficulties are intrinsically associated with increased morbidity and mortality in those who suffer from it [9] as was the case presented here. Our patient presented with brain and pulmonary tuberculosis, with cerebral stroke.
Central nervous system tuberculosis (CNS TB) is associated with high mortality, it can occur in 1% to 5% of all patients with TB and 10% of those with AIDS-related TB [10].
Active TB disease can enhance HIV replication and consequently accelerate the destruction of CD4 T lymphocytes and the course of HIV infection [11] . On admission, only fever, which had been present for 7 days, as well as cough for the past two day were the only clinical manifestations. Chest radiograph suggested miliary TB. This diagnosis was confirmed when multi susceptible M. tuberculosis was identified in respiratory secretion. On clinical findings, the patient was immediately administered anti-TB therapy which resulted being effective, based on successive susceptibility test results. The same day, in the absence of neurologic manifestations, CSF was examined evidencing features consistent with probable tuberculous meningitis: pleiocytosis, white cell count 150 cell mm3 , increased protein level and low glucose concentration [12].
The actual mechanisms contributing to the association between tuberculosis and stroke are not fully understood. Infections by Mycobacterium tuberculosis lead to the activation of a persistent inflammatory response that starts a cascade of cytokines and chemokines [13] Inflammatory response has been established to be of pathogenic relevance in the link between infection and atherosclerosis, and it may relate to endothelial dysfunction inflicted by bacterial endotoxins and the action of cytokines [14]. Increased C-reactive protein, a marker of systemic inflammation accompanying active tuberculosis, may also be associated with atherosclerosis and could lead to cardiovascular events [15]. Another mechanism that links infection with atherosclerosis may be the induction of autoimmunity through antibodies.
Infections may also cause fever, dehydration, altered coagulation and fibrinolysis balance, and platelet activation, all of which increase the risk of stroke [16]. Moreover, recent respiratory tract infections increase the risk for cardioembolic and large-vessel atherothromboembolic stroke subtypes, and pulmonary tuberculosis may increase the stroke risk mediated by secondary respiratory tract infections [17].
As human immunodeficiency virus emerges as a key factor undermining global tuberculosis control, coinfection with M tuberculosis may increase the infectious burden, augmenting the risk of stroke fostered by tuberculosis [18]. In our case it was not a contraindication for the administration of aspirin.
Cerebrospinal fluid examination is the cornerstone of TBM diagnosis and the gold standard is the identification of M. tuberculosis in the CSF [19,21].
The value of anti-inflammatory treatment with corticosteroids [22] in preventing infarction in TBM is controversial and the role on anti-TB therapy on the development of stroke is also not well defined [20].
CONCLUSION
Miliary tuberculosis is a hematogenous dissemination of Mycobacterium Tuberculosis which involves especially lungs, central nervous system and lymph node. An acute cerebrovascular event may occur in the course of an underlying disease, and the infection may be the etiological cause of cerebral infarction when associated with other underlying infectious conditions.TBM must be included in the differential diagnosis of HIV infected patients with stroke and TBM treatment needs be started as soon as possible before the onset of vasculopathy.
CONFLICT OF INTEREST: None.
REFERENCE
| 1. CDCTB. Data and Statistics . (2023). Accessed: July 3, 2023: https://www.cdc.gov/tb/statistics/default.htm |
| 2. World Health Organization: Global TB report 2017. WHO; 2017. http://apps. who.int/iris/bitstream/10665/259366/1/9789241565516-eng.pdf? |
| 3. Gropper MR, Schulder M, Sharan AD et al: Central nervous system tuberculosis: Medical management and surgical indications. Surg Neurol, 1995; 44(4): 378–84 |
| 4. Rock RB, Olin M, Baker CA, Molitor TW, Peterson PK. Central nervous system tuberculosis and clinical aspects. Clin Microbiol Rev. 2008; 21: 243-261. |
| 5. Misra UK, Kalita J, Maurya PK. Stroke in tuberculous meningitis. J Neurol Sci. 2011; 303: 22-30. |
| 6. Lammie GA, Hewlett RH, Schoeman JF, Donald PR. Tuberculous cerebrovascular disease. A review. J Infect. 2009; 59: 156-166. |
| 7. Vinnard C, Macgregor RR. Tuberculous meningitis in HIV infected individuals. Curr HIV/AIDS Rep. 2009; 6 (3): 139-145. |
| 8. Sheu J-J, Chiou H-Y, Kang J-H, Chen Y-H, Lin H-C. Tubeculosis and risk of ischemic stroke. A 3 year follow-up study. Stroke. 2010; 41: 244-249. |
| 9. Maheswari EU, Bhoopathy RM, Bhanu K et al: Clinical spectrum of central nervous system tuberculosis and the efficacy of revised national tuberculosis control program in its management. J Neurosci Rural Pract, 2011; 10(1): 71–77. |
| 10. Raviglione MC, Snider DE Jr., Kochi A: Global epidemiology of tuberculosis. Morbidity and mortality of a worldwide epidemic. JAMA, 1995; 273(3): 220–26. |
| 11. Garg RK, Sinha MK. Tuberculous meningitis in patients infected with human immunodeficiency virus. J Neurol. 2011; 258: 3-13. |
| 12. Thwaites GE, Fisher M, Hemingway C, Scott G, Solomon T, Innes J. British infection society guidelines for diagnosis and treatment of tuberculosis of the central nervous system in adults and children. J Infect. 2009; 59: 167-187. |
| 13. Kaplan G, Freedman VH. The role of cytokines in the immune response to tuberculosis. Res Immunol. 1996;147:565–572. |
| 14. Libby P, Egan D. Skarlatos S. Roles of infectious agents in atherosclerosis and restenosis: an assessment of the evidence and need for future research. Circulation. 1997;96:4095– 4103. |
| 15. Lindsberg PJ, Grau AJ. Inflammation and infections as risk factors for ischemic stroke. Stroke. 2003;34:2518 –2532. |
| 16. Emsley HC, Hopkins SJ. Acute ischaemic stroke and infection: recent and emerging concepts. Lancet Neurol. 2008;7:341–353. |
| 17. Grau AJ, Fischer B, Barth C, Ling P, Lichy C, Buggle F. Influenza vaccination is associated with a reduced risk of stroke. Stroke. 2005;36: 1501–1506. |
| 18. Espinola-Klein C, Rupprecht HJ, Blankenberg S, Bickel C, Kopp H, Rippin G, Victor A, Hafner G, Schlumberger W, Meyer J, AtheroGene Investigators. Impact of infectious burden on extent and long-term prognosis of atherosclerosis. Circulation. 2002;105:15–21. |
| 19. Rock RB, Olin M, Baker CA, Molitor TW, Peterson PK. Central nervous system tuberculosis and clinical aspects. Clin Microbiol Rev. 2008; 21: 243-261. http://dx.doi.org/10.1128/CMR.00042- 07 PMid:18400795 PMCid:2292571. |
| 20. Garg RK, Sinha MK. Tuberculous meningitis in patients infected with human immunodeficiency virus. J Neurol. 2011; 258: 3-13 |
| 21. E Lee M-H, Yang H-I, Wang C-H, Jen C-L, Yeh S-H, Liu C-J, You S-L, Chen WJ, Chen C-J. Hepatits C virus infection and increased risk of cerebrovascular diseases. Stroke. 2010; 41: 2894-2900. |
| 22. Marais S, Thwaites G, Schoeman JF, Misra UK, Prasad K, Donald P, Wilkinson RJ, Marais BJ. Tuberculous meningitis: a uniform case definition for use in clinical research. Lancet. 2010; 10: 803-812. |
Figures
REFERENCE
| 1. CDCTB. Data and Statistics . (2023). Accessed: July 3, 2023: https://www.cdc.gov/tb/statistics/default.htm |
| 2. World Health Organization: Global TB report 2017. WHO; 2017. http://apps. who.int/iris/bitstream/10665/259366/1/9789241565516-eng.pdf? |
| 3. Gropper MR, Schulder M, Sharan AD et al: Central nervous system tuberculosis: Medical management and surgical indications. Surg Neurol, 1995; 44(4): 378–84 |
| 4. Rock RB, Olin M, Baker CA, Molitor TW, Peterson PK. Central nervous system tuberculosis and clinical aspects. Clin Microbiol Rev. 2008; 21: 243-261. |
| 5. Misra UK, Kalita J, Maurya PK. Stroke in tuberculous meningitis. J Neurol Sci. 2011; 303: 22-30. |
| 6. Lammie GA, Hewlett RH, Schoeman JF, Donald PR. Tuberculous cerebrovascular disease. A review. J Infect. 2009; 59: 156-166. |
| 7. Vinnard C, Macgregor RR. Tuberculous meningitis in HIV infected individuals. Curr HIV/AIDS Rep. 2009; 6 (3): 139-145. |
| 8. Sheu J-J, Chiou H-Y, Kang J-H, Chen Y-H, Lin H-C. Tubeculosis and risk of ischemic stroke. A 3 year follow-up study. Stroke. 2010; 41: 244-249. |
| 9. Maheswari EU, Bhoopathy RM, Bhanu K et al: Clinical spectrum of central nervous system tuberculosis and the efficacy of revised national tuberculosis control program in its management. J Neurosci Rural Pract, 2011; 10(1): 71–77. |
| 10. Raviglione MC, Snider DE Jr., Kochi A: Global epidemiology of tuberculosis. Morbidity and mortality of a worldwide epidemic. JAMA, 1995; 273(3): 220–26. |
| 11. Garg RK, Sinha MK. Tuberculous meningitis in patients infected with human immunodeficiency virus. J Neurol. 2011; 258: 3-13. |
| 12. Thwaites GE, Fisher M, Hemingway C, Scott G, Solomon T, Innes J. British infection society guidelines for diagnosis and treatment of tuberculosis of the central nervous system in adults and children. J Infect. 2009; 59: 167-187. |
| 13. Kaplan G, Freedman VH. The role of cytokines in the immune response to tuberculosis. Res Immunol. 1996;147:565–572. |
| 14. Libby P, Egan D. Skarlatos S. Roles of infectious agents in atherosclerosis and restenosis: an assessment of the evidence and need for future research. Circulation. 1997;96:4095– 4103. |
| 15. Lindsberg PJ, Grau AJ. Inflammation and infections as risk factors for ischemic stroke. Stroke. 2003;34:2518 –2532. |
| 16. Emsley HC, Hopkins SJ. Acute ischaemic stroke and infection: recent and emerging concepts. Lancet Neurol. 2008;7:341–353. |
| 17. Grau AJ, Fischer B, Barth C, Ling P, Lichy C, Buggle F. Influenza vaccination is associated with a reduced risk of stroke. Stroke. 2005;36: 1501–1506. |
| 18. Espinola-Klein C, Rupprecht HJ, Blankenberg S, Bickel C, Kopp H, Rippin G, Victor A, Hafner G, Schlumberger W, Meyer J, AtheroGene Investigators. Impact of infectious burden on extent and long-term prognosis of atherosclerosis. Circulation. 2002;105:15–21. |
| 19. Rock RB, Olin M, Baker CA, Molitor TW, Peterson PK. Central nervous system tuberculosis and clinical aspects. Clin Microbiol Rev. 2008; 21: 243-261. http://dx.doi.org/10.1128/CMR.00042- 07 PMid:18400795 PMCid:2292571. |
| 20. Garg RK, Sinha MK. Tuberculous meningitis in patients infected with human immunodeficiency virus. J Neurol. 2011; 258: 3-13 |
| 21. E Lee M-H, Yang H-I, Wang C-H, Jen C-L, Yeh S-H, Liu C-J, You S-L, Chen WJ, Chen C-J. Hepatits C virus infection and increased risk of cerebrovascular diseases. Stroke. 2010; 41: 2894-2900. |
| 22. Marais S, Thwaites G, Schoeman JF, Misra UK, Prasad K, Donald P, Wilkinson RJ, Marais BJ. Tuberculous meningitis: a uniform case definition for use in clinical research. Lancet. 2010; 10: 803-812. |
ARTICLE INFO DOI: 10.12699/jfvpulm.15.45.2024.62
Conflict of Interest
Non
Date of manuscript receiving
05/12/2023
Date of publication after correction
24/01/2024
Article citation
Mohamed Amine Mnaili, Ahmed Bourazza. Ischemic stroke in a patient with tuberculous meningitis and HIV infection. J Func Vent Pulm 2024;45(15):62-65