
 English
English
 French
French
Effectiveness of Sleep Lab (Lavender) Spray in Improving Sleep Quality in Insomnia Patients
Efficacité du spray Sleep Lab (Lavender) dans l’amélioration de la qualité du sommeil chez les patients insomniaques
Tang-Thi-Thao Tram1,3, Tran-Thi-Cam Tu3 ,Nguyen-Tuan Anh1,3, Sy Duong-Quy 1,2,3
1 Lam Dong Medical College
2 Penn State Medical College. Hershey Medical Center. PA, USA
3 Sleep Research Center. Vietnam Society of Sleep Medicine. Lam Dong, Vietnam
Corresponding Author:
Tang Thi Thao Tram
Lam Dong Medical College. Dalat city. Vietnam
ABSTRACT
Background: Insomnia is a highly prevalent sleep disorder associated with impaired daytime functioning and multiple comorbidities. Non-pharmacological interventions, including aromatherapy, have attracted growing attention due to their safety and accessibility. This study evaluated the effectiveness of Sleep Lab (Lavender) spray in improving sleep quality among patients with chronic insomnia. Methods: A total of 32 patients with chronic insomnia (mean age 50.5 ± 14.6 years; 65.6% female) were enrolled. Baseline demographic, lifestyle, and clinical characteristics were collected. Subjective sleep outcomes were assessed using the Epworth Sleepiness Scale (ESS), daytime sleepiness, fatigue severity, and self-reported sleep quality scores. Objective sleep parameters were evaluated via overnight polysomnography. Participants received Sleep Lab (Lavender) spray for three weeks, and outcomes were compared between baseline and post-intervention. Results: At baseline, participants reported markedly reduced nighttime sleep duration (3.5 ± 1.0 hours), poor sleep quality (71.9%), and high rates of severe insomnia (75%). After three weeks of intervention, ESS scores improved significantly (10.6 to 2.8; p < 0.001). Subjective outcomes showed reductions in daytime sleepiness (6.1 ± 1.8 to 2.7 ± 1.5; p < 0.001) and fatigue severity (7.7 ± 1.4 to 2.8 ± 1.3; p < 0.001), along with improved sleep quality (2.7 ± 1.8 to 6.8 ± 1.3; p < 0.001). Polysomnographic findings revealed a substantial increase in deep sleep (N3: 6.53% to 34.52%), reductions in light sleep stages (N1: 9.58% to 4.93%; N2: 55.63% to 39.21%), and a decrease in arousal index (39 to 17 events/hour). Conclusion: Sleep Lab (Lavender) spray significantly improved both subjective and objective sleep outcomes in patients with chronic insomnia. The intervention enhanced deep sleep, reduced fatigue, and improved sleep continuity. Given its favorable safety profile and ease of use, lavender spray may serve as a valuable adjunctive or alternative therapy for chronic insomnia management.
KEYWORDS: insomnia, lavender, aromatherapy, sleep quality, polysomnography
RÉSUMÉ
Contexte : L’insomnie est un trouble du sommeil très répandu, associé à une altération du fonctionnement diurne et à de multiples comorbidités. Les interventions non pharmacologiques, y compris l’aromathérapie, suscitent un intérêt croissant en raison de leur sécurité et de leur accessibilité. Cette étude a évalué l’efficacité du spray Sleep Lab (Lavande) dans l’amélioration de la qualité du sommeil chez des patients souffrant d’insomnie chronique. Méthodes : Un total de 32 patients atteints d’insomnie chronique (âge moyen 50,5 ± 14,6 ans ; 65,6 % de femmes) ont été inclus. Les caractéristiques démographiques, de mode de vie et cliniques de base ont été recueillies. Les résultats subjectifs liés au sommeil ont été évalués à l’aide de l’échelle de somnolence d’Epworth (ESS), de la somnolence diurne, de la sévérité de la fatigue et des scores de qualité du sommeil auto-déclarés. Les paramètres objectifs du sommeil ont été mesurés par polysomnographie nocturne. Les participants ont reçu le spray Sleep Lab (Lavande) pendant trois semaines, et les résultats ont été comparés entre la période initiale et la période post-intervention. Résultats : Au départ, les participants rapportaient une durée de sommeil nocturne fortement réduite (3,5 ± 1,0 heures), une mauvaise qualité du sommeil (71,9 %) et des taux élevés d’insomnie sévère (75 %). Après trois semaines d’intervention, les scores ESS se sont significativement améliorés (10,6 à 2,8 ; p < 0,001). Les résultats subjectifs ont montré une diminution de la somnolence diurne (6,1 ± 1,8 à 2,7 ± 1,5 ; p < 0,001) et de la sévérité de la fatigue (7,7 ± 1,4 à 2,8 ± 1,3 ; p < 0,001), ainsi qu’une amélioration de la qualité du sommeil (2,7 ± 1,8 à 6,8 ± 1,3 ; p < 0,001). Les résultats polysomnographiques ont révélé une augmentation substantielle du sommeil profond (N3 : 6,53 % à 34,52 %), une réduction des stades de sommeil léger (N1 : 9,58 % à 4,93 % ; N2 : 55,63 % à 39,21 %), et une diminution de l’index d’éveils (39 à 17 événements/heure).
Conclusion : Le spray Sleep Lab (Lavande) a significativement amélioré les résultats subjectifs et objectifs du sommeil chez les patients souffrant d’insomnie chronique. L’intervention a favorisé le sommeil profond, réduit la fatigue et amélioré la continuité du sommeil. Compte tenu de son profil de sécurité favorable et de sa facilité d’utilisation, le spray à la lavande pourrait constituer une thérapie adjuvante ou alternative précieuse dans la prise en charge de l’insomnie chronique.
MOTS CLÉS: insomnie, lavande, aromathérapie, qualité du sommeil, polysomnographie
INTRODUCTION
Insomnia is one of the most common sleep disorders worldwide, characterized by difficulty initiating or maintaining sleep, early morning awakenings, and impaired daytime functioning. The global prevalence of insomnia symptoms is estimated to range from 10% to 30%, with higher rates reported among women and older adults [1,2]. Chronic insomnia is associated with substantial adverse consequences, including cognitive decline, reduced work productivity, impaired quality of life, and an increased risk of cardiovascular, metabolic, and psychiatric disorders [3–5].
Pharmacological treatments, such as benzodiazepines and non-benzodiazepine hypnotics, are commonly prescribed for insomnia. Although effective in the short term, these medications are associated with tolerance, dependence, next-day residual effects, and potential long-term health risks [6]. Non-pharmacological approaches, particularly cognitive behavioral therapy for insomnia (CBT-I), are recommended as first-line treatment. However, CBT-I requires trained professionals, multiple sessions, and may not be readily accessible in many settings [7]. This has prompted growing interest in complementary and alternative therapies for insomnia.
Lavender (Lavandula angustifolia) is one of the most widely used medicinal plants in aromatherapy. Experimental and clinical evidence suggests that lavender exerts anxiolytic, sedative, and sleep-promoting effects through modulation of the autonomic nervous system and gamma-aminobutyric acid (GABA) pathways [8,9]. Previous randomized controlled trials have demonstrated that lavender aromatherapy can improve subjective sleep quality and reduce symptoms of insomnia [10,11]. However, most studies have focused on inhalation or capsule formulations, with limited evidence on the efficacy of lavender sprays as a practical, user-friendly delivery method.
Therefore, this study aimed to evaluate the effectiveness of Sleep Lab (Lavender) spray in improving sleep quality, daytime symptoms, and sleep architecture in patients with chronic insomnia. We hypothesized that lavender spray would significantly enhance both subjective and objective sleep outcomes after three weeks of intervention.
Methods
Study Design
This was a randomized controlled clinical trial designed to evaluate the effectiveness of Sleep Lab (Lavender) spray in patients with insomnia. A total of 32 patients diagnosed with insomnia were enrolled, and the diagnosis was confirmed objectively through polysomnography (PSG). This design ensured accuracy in diagnosis and reliability in evaluating treatment efficacy.
Participants and Eligibility Criteria
Eligible participants were adults aged 18–65 years who were diagnosed with insomnia based on polysomnographic findings. Patients were excluded if they had a history of using sedatives or other sleep-related therapies within four weeks prior to enrollment.
Intervention
Participants were instructed to use Sleep Lab (Lavender) spray once daily for three consecutive weeks. The spray was applied approximately 30 minutes before bedtime according to the manufacturer’s recommendations.
Study Procedures
Patients presenting with sleep complaints underwent polysomnography.
Those not diagnosed with insomnia were excluded.
Patients confirmed with insomnia were enrolled, interviewed using the Pittsburgh Sleep Quality Index (PSQI), and instructed on proper use of Sleep Lab spray.
After three weeks of intervention, participants underwent repeat polysomnography.
Data were collected and analyzed for treatment outcomes.
Outcome Measures
The primary outcomes included:
Total Sleep Time (TST)
Sleep Onset Latency (SOL)
Sleep quality, assessed by polysomnography and the Pittsburgh Sleep Quality Index (PSQI).
Statistical Analysis
Data were analyzed using SPSS version 22.0 (IBM Corp., Armonk, NY, USA). Paired t-tests were used to compare pre- and post-intervention results. A p-value of <0.05 was considered statistically significant. The study posed no harm to patients or their families. All participants and their relatives were informed about the study objectives and procedures, and participation was voluntary. Patients who declined participation were not discriminated against in their treatment or follow-up. All collected information was kept strictly confidential. Data were recorded honestly, and results were analyzed according to scientific principles.
Results
Baseline Characteristics
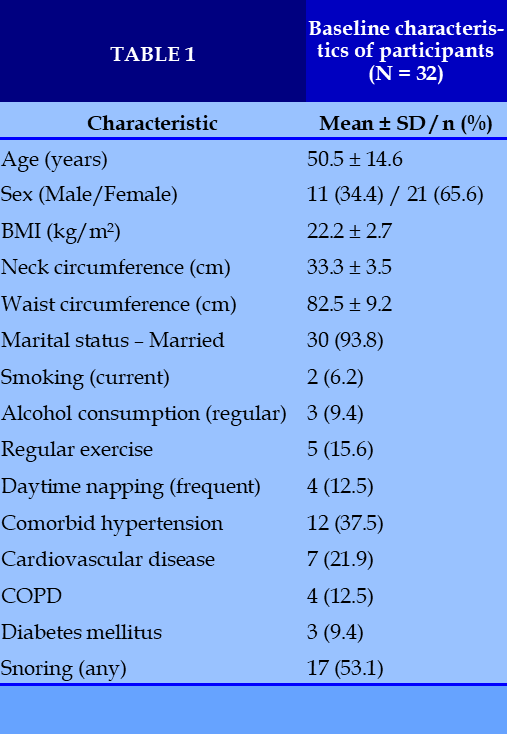
A total of 32 patients with chronic insomnia were included in the study. The mean age was 50.5 ± 14.6 years, indicating that most participants were middle-aged or older adults. Females accounted for 65.6% of the cohort, reflecting the higher prevalence of insomnia among women, particularly around menopause.
Anthropometric measurements showed that the mean BMI was 22.2 ± 2.7 kg/m², within the normal range. The mean neck and waist circumferences were 33.3 ± 3.5 cm and 82.5 ± 9.2 cm, respectively, suggesting that obesity-related risk factors such as central adiposity or obstructive sleep apnea were not predominant in this sample.
Regarding marital status, 93.8% of participants were married, while only 6.3% were divorced. Lifestyle assessment revealed that the majority did not smoke (93.8%) or consume alcohol regularly (90.6%). However, electronic device use before bedtime was frequent, with 50% reporting occasional and 25% frequent use. Regular exercise was reported by 15.6% of participants, while 53.1% exercised occasionally. Prolonged daytime napping was also common, with 56.3% reporting this habit occasionally or frequently.
With respect to comorbidities, hypertension (37.5%) and cardiovascular diseases (21.9%) were the most prevalent conditions, followed by chronic obstructive pulmonary disease (12.5%) and diabetes mellitus (9.4%). Neurological disorders (6.3%) and stroke (3.1%) were less common. Snoring was present in 53.1% of patients, with 34.4% classified as severe; in 40.6% of cases, snoring was reported to disturb bed partners. (Table 1)
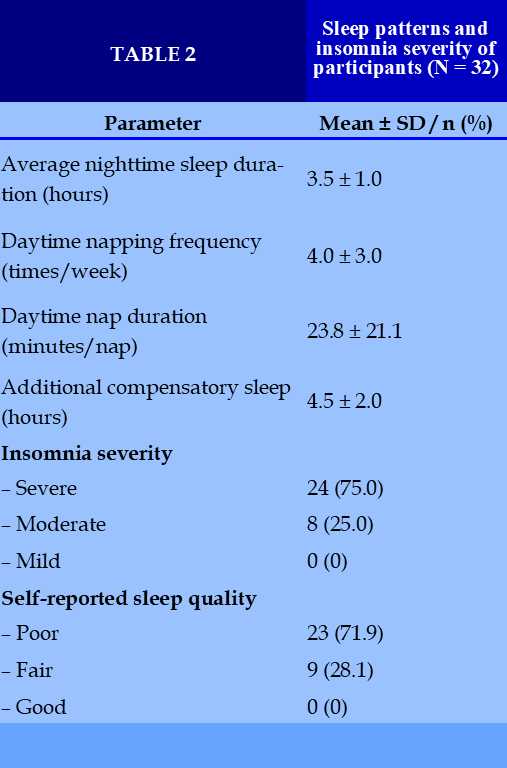
Sleep Patterns and Insomnia Severity
Participants had a markedly reduced average sleep duration of 3.5 ± 1.0 hours per night. Daytime napping occurred an average of 4.0 ± 3.0 times per week, lasting 23.8 ± 21.1 minutes per nap, with an additional 4.5 ± 2.0 hours of compensatory sleep outside of nighttime rest.
All patients (100%) met criteria for insomnia, with 75% reporting severe and 25% moderate symptoms. None rated their sleep quality as good; 28.1% described it as fair, while 71.9% reported poor sleep quality. (Table 2)
Effects of Sleep Lab (Lavender) Spray
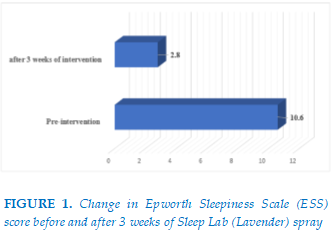
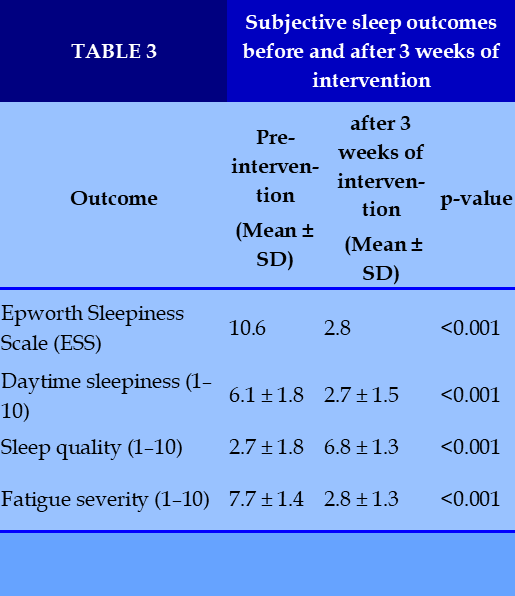
After 3 weeks of intervention with Sleep Lab (Lavender) spray, significant improvements were observed. The mean Epworth Sleepiness Scale (ESS) score decreased from 10.6 at baseline (moderate daytime sleepiness) to 2.8 post-intervention (minimal sleepiness). (Figure 1)
Subjective outcomes showed marked improvements across multiple domains. Daytime sleepiness decreased from 6.1 ± 1.8 to 2.7 ± 1.5 (p < 0.001), sleep quality improved from 2.7 ± 1.8 to 6.8 ± 1.3 (p < 0.001), and fatigue severity was reduced from 7.7 ± 1.4 to 2.8 ± 1.3 (p < 0.001). (Table 3)
Polysomnographic Findings
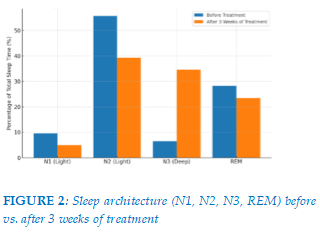
Objective sleep assessment revealed favorable changes in sleep architecture. After 3 weeks of treatment, the proportion of deep sleep (N3) increased substantially from 6.53% to 34.52%, while light sleep stages decreased (N1: 9.58% to 4.93%; N2: 55.63% to 39.21%). REM sleep showed a modest reduction (28.26% to 23.49%). (Figure 2)
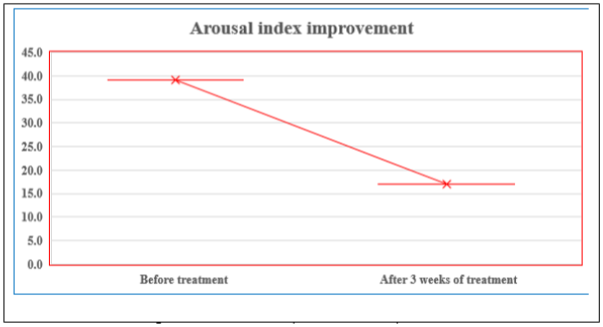
The arousal index also improved markedly, decreasing from 39 to 17 events per hour, indicating a significant reduction in sleep fragmentation and enhanced sleep continuity. (Figure 3)
Discussion
This study demonstrated that the Sleep Lab (Lavender) spray significantly improved both subjective and objective sleep parameters among patients with chronic insomnia. After three weeks of intervention, participants reported substantial reductions in daytime sleepiness and fatigue, along with improved sleep quality. These findings were corroborated by polysomnographic data showing a marked increase in deep sleep (N3) and a reduction in sleep fragmentation, as indicated by the lower arousal index.
The observed benefits are consistent with previous evidence highlighting the therapeutic effects of lavender on sleep and relaxation. Lavender essential oil has been shown to exert anxiolytic and sedative properties, primarily through modulation of the autonomic nervous system and gamma-aminobutyric acid (GABA) receptors [12]. Several randomized controlled trials have demonstrated that lavender aromatherapy can enhance sleep quality and reduce insomnia severity [13,14]. Our results align with these studies, suggesting that lavender spray may be a convenient, non-pharmacological option for insomnia management.
The increase in deep sleep (N3) observed in this study is particularly noteworthy. Deep sleep plays a crucial role in memory consolidation, immune regulation, and overall restorative processes [15]. Insomnia patients often experience reduced slow-wave sleep, which contributes to fatigue and impaired daytime functioning [16]. By enhancing N3 duration, lavender spray may help restore these physiological functions, offering a potential advantage over conventional pharmacological agents that primarily reduce sleep latency without significantly improving sleep architecture.
Another important finding was the improvement in the Epworth Sleepiness Scale (ESS), decreasing from moderate to minimal sleepiness levels. Excessive daytime sleepiness is a common complaint among insomnia patients and is associated with reduced work productivity, impaired cognitive performance, and increased risk of accidents [17]. Our findings suggest that lavender spray not only improves nocturnal sleep but also alleviates daytime consequences of insomnia, thereby enhancing overall quality of life.
The reduction in arousal index from 39 to 17 events per hour also deserves emphasis. Frequent arousals are a hallmark of poor sleep continuity and have been linked to cardiovascular and metabolic dysfunctions [18,20]. By improving sleep stability, lavender spray may exert additional protective effects beyond sleep restoration.
Despite these promising results, several limitations must be acknowledged. The relatively small sample size and short intervention period limit the generalizability of our findings. Moreover, the absence of a placebo control prevents us from fully excluding expectancy effects. Future large-scale, randomized controlled trials with longer follow-up are warranted to confirm the efficacy and safety of lavender spray in diverse populations.
In conclusion, this study provides evidence that Sleep Lab (Lavender) spray is effective in improving sleep quality, reducing fatigue, and enhancing deep sleep in patients with chronic insomnia. Given its non-invasive and well-tolerated nature, lavender spray may serve as a valuable adjunctive or alternative therapy to conventional pharmacological treatments.
REFERENCES
| 1. Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev. 2002;6(2):97–111. |
| 2. Morin CM, Benca R. Chronic insomnia. Lancet. 2012;379(9821):1129–41. |
| 3. Riemann D, Baglioni C, Bassetti C, et al. European guideline for the diagnosis and treatment of insomnia. J Sleep Res. 2017;26(6):675–700. |
| 4. Léger D, Bayon V. Societal costs of insomnia. Sleep Med Rev. 2010;14(6):379–89. |
| 5. Laugsand LE, Strand LB, Platou C, Vatten LJ, Janszky I. Insomnia and the risk of incident heart failure: a population study. Eur Heart J. 2014;35(21):1382–93. |
| 6. Glass J, Lanctôt KL, Herrmann N, Sproule BA, Busto UE. Sedative hypnotics in older people with insomnia: meta-analysis of risks and benefits. BMJ. 2005;331(7526):1169. |
| 7. Trauer JM, Qian MY, Doyle JS, Rajaratnam SM, Cunnington D. Cognitive behavioral therapy for chronic insomnia: a systematic review and meta-analysis. Ann Intern Med. 2015;163(3):191–204. |
| 8. Koulivand PH, Khaleghi Ghadiri M, Gorji A. Lavender and the nervous system. Evid Based Complement Alternat Med. 2013;2013:681304. |
| 9. Kritsidima M, Newton T, Asimakopoulou K. The effects of lavender scent on dental patient anxiety levels: a cluster randomised-controlled trial. Community Dent Oral Epidemiol. 2010;38(1):83–7. |
| 10. Hwang JH, Shin JW, Lee JS, Lee YJ. Effects of lavender aroma on sleep quality in patients with self-reported insomnia. J Altern Complement Med. 2015;21(3):173–9. |
| 11. Lillehei AS, Halcon LL, Savik K, Reis R. Effect of inhaled lavender and sleep hygiene on self-reported sleep issues: a randomized controlled trial. J Altern Complement Med. 2015;21(7):430–8. |
| 12. Koulivand PH, Khaleghi Ghadiri M, Gorji A. Lavender and the nervous system. Evid Based Complement Alternat Med. 2013;2013:681304. |
| 13. Hwang JH, Shin JW, Lee JS, Lee YJ. Effects of lavender aroma on sleep quality in patients with self-reported insomnia. J Altern Complement Med. 2015;21(3):173–9. |
| 14. Lillehei AS, Halcon LL, Savik K, Reis R. Effect of inhaled lavender and sleep hygiene on self-reported sleep issues: a randomized controlled trial. J Altern Complement Med. 2015;21(7):430–8. |
| 15. Mander BA, Winer JR, Walker MP. Sleep and human aging. Neuron. 2017;94(1):19–36. |
| 16. Riemann D, Baglioni C, Bassetti C, et al. European guideline for the diagnosis and treatment of insomnia. J Sleep Res. 2017;26(6):675–700. |
| 17. Léger D, Bayon V. Societal costs of insomnia. Sleep Med Rev. 2010;14(6):379–89. |
| 18. Javaheri S, Redline S. Insomnia and risk of cardiovascular disease. Chest. 2017;152(2):435–44. |
| 19. Fernández-San-Martín MI, Masa-Font R, Palacios-Soler L, Sancho-Gómez P, Calbó-Caldentey C, Flores-Mateo G. Effectiveness of Valerian on insomnia: a meta-analysis of randomized placebo-controlled trials. Sleep Med. 2010;11(6):505–11. |
| 20. Laugsand LE, Strand LB, Platou C, Vatten LJ, Janszky I. Insomnia and the risk of incident heart failure: a population study. Eur Heart J. 2014;35(21):1382–93. |
| 21. Dang-Thi-Mai K, Dang-Vu T, Tran-Van N, Le-Thuong V, Vu H, Duong-Quy S. Study on the correlation between excessive daytime sleepiness and obstructive sleep apnea. J Func Vent Pulm. 2020;11(34):28–32. |
| 22. Bui-Diem K, Do-Van D, Duong-Quy S. Methods for assessing alertness and level of vigilance in patients with obstructive sleep apnea. J Func Vent Pulm. 2020;11(35):1–6. |
| 23. Duong-Quy S, Tran-Duc S, Hoang-Chau-Bao D, Bui-Diem K, Vu-Tran-Thien Q, Nguyen-Nhu V. Tiredness, depression, and sleep disorders in frontline healthcare workers during COVID-19 pandemic in Vietnam: A field hospital study. Front Psychiatry. 2022 Oct 17;13:984658. |
| 24. Bui-Diem K, Hung C-H, Zhu G-C, Nguyen-Van T, Nguyen-Binh T, Vu-Tran-Thien Q, To-Truong D, Ngo-Thanh H, Duong-Quy S. Physical therapy for sleep apnea: a smartphone application for home-based physical therapy for patients with obstructive sleep apnea. Front Neurol. 2023 May 25;14:1124059. |
| 25. Mai NT-Phuong, Mai NT-Thanh, Goldberg RJ, Nguyen HL, Dao T-Minh A, Duong-Quy S. Obstructive Sleep Apnea and Sleep Disorders in Children with Attention Deficit Hyperactivity Disorder. Pulm Ther. 2025 Jul 7;1–19. |
FIGURES - TABLES
REFERENCES
| 1. Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev. 2002;6(2):97–111. |
| 2. Morin CM, Benca R. Chronic insomnia. Lancet. 2012;379(9821):1129–41. |
| 3. Riemann D, Baglioni C, Bassetti C, et al. European guideline for the diagnosis and treatment of insomnia. J Sleep Res. 2017;26(6):675–700. |
| 4. Léger D, Bayon V. Societal costs of insomnia. Sleep Med Rev. 2010;14(6):379–89. |
| 5. Laugsand LE, Strand LB, Platou C, Vatten LJ, Janszky I. Insomnia and the risk of incident heart failure: a population study. Eur Heart J. 2014;35(21):1382–93. |
| 6. Glass J, Lanctôt KL, Herrmann N, Sproule BA, Busto UE. Sedative hypnotics in older people with insomnia: meta-analysis of risks and benefits. BMJ. 2005;331(7526):1169. |
| 7. Trauer JM, Qian MY, Doyle JS, Rajaratnam SM, Cunnington D. Cognitive behavioral therapy for chronic insomnia: a systematic review and meta-analysis. Ann Intern Med. 2015;163(3):191–204. |
| 8. Koulivand PH, Khaleghi Ghadiri M, Gorji A. Lavender and the nervous system. Evid Based Complement Alternat Med. 2013;2013:681304. |
| 9. Kritsidima M, Newton T, Asimakopoulou K. The effects of lavender scent on dental patient anxiety levels: a cluster randomised-controlled trial. Community Dent Oral Epidemiol. 2010;38(1):83–7. |
| 10. Hwang JH, Shin JW, Lee JS, Lee YJ. Effects of lavender aroma on sleep quality in patients with self-reported insomnia. J Altern Complement Med. 2015;21(3):173–9. |
| 11. Lillehei AS, Halcon LL, Savik K, Reis R. Effect of inhaled lavender and sleep hygiene on self-reported sleep issues: a randomized controlled trial. J Altern Complement Med. 2015;21(7):430–8. |
| 12. Koulivand PH, Khaleghi Ghadiri M, Gorji A. Lavender and the nervous system. Evid Based Complement Alternat Med. 2013;2013:681304. |
| 13. Hwang JH, Shin JW, Lee JS, Lee YJ. Effects of lavender aroma on sleep quality in patients with self-reported insomnia. J Altern Complement Med. 2015;21(3):173–9. |
| 14. Lillehei AS, Halcon LL, Savik K, Reis R. Effect of inhaled lavender and sleep hygiene on self-reported sleep issues: a randomized controlled trial. J Altern Complement Med. 2015;21(7):430–8. |
| 15. Mander BA, Winer JR, Walker MP. Sleep and human aging. Neuron. 2017;94(1):19–36. |
| 16. Riemann D, Baglioni C, Bassetti C, et al. European guideline for the diagnosis and treatment of insomnia. J Sleep Res. 2017;26(6):675–700. |
| 17. Léger D, Bayon V. Societal costs of insomnia. Sleep Med Rev. 2010;14(6):379–89. |
| 18. Javaheri S, Redline S. Insomnia and risk of cardiovascular disease. Chest. 2017;152(2):435–44. |
| 19. Fernández-San-Martín MI, Masa-Font R, Palacios-Soler L, Sancho-Gómez P, Calbó-Caldentey C, Flores-Mateo G. Effectiveness of Valerian on insomnia: a meta-analysis of randomized placebo-controlled trials. Sleep Med. 2010;11(6):505–11. |
| 20. Laugsand LE, Strand LB, Platou C, Vatten LJ, Janszky I. Insomnia and the risk of incident heart failure: a population study. Eur Heart J. 2014;35(21):1382–93. |
| 21. Dang-Thi-Mai K, Dang-Vu T, Tran-Van N, Le-Thuong V, Vu H, Duong-Quy S. Study on the correlation between excessive daytime sleepiness and obstructive sleep apnea. J Func Vent Pulm. 2020;11(34):28–32. |
| 22. Bui-Diem K, Do-Van D, Duong-Quy S. Methods for assessing alertness and level of vigilance in patients with obstructive sleep apnea. J Func Vent Pulm. 2020;11(35):1–6. |
| 23. Duong-Quy S, Tran-Duc S, Hoang-Chau-Bao D, Bui-Diem K, Vu-Tran-Thien Q, Nguyen-Nhu V. Tiredness, depression, and sleep disorders in frontline healthcare workers during COVID-19 pandemic in Vietnam: A field hospital study. Front Psychiatry. 2022 Oct 17;13:984658. |
| 24. Bui-Diem K, Hung C-H, Zhu G-C, Nguyen-Van T, Nguyen-Binh T, Vu-Tran-Thien Q, To-Truong D, Ngo-Thanh H, Duong-Quy S. Physical therapy for sleep apnea: a smartphone application for home-based physical therapy for patients with obstructive sleep apnea. Front Neurol. 2023 May 25;14:1124059. |
| 25. Mai NT-Phuong, Mai NT-Thanh, Goldberg RJ, Nguyen HL, Dao T-Minh A, Duong-Quy S. Obstructive Sleep Apnea and Sleep Disorders in Children with Attention Deficit Hyperactivity Disorder. Pulm Ther. 2025 Jul 7;1–19. |
ARTICLE INFO DOI: 10.12699/jfvpulm.suppl.16.50.2025.19
Conflict of Interest
Non
Date of manuscript receiving
20/05/2025
31/08/2025
Article citation
Tang-Thi-Thao Tram, Tran-Thi-Cam Tu, Nguyen-Tuan Anh, Sy Duong-Quy. Effectiveness of Sleep Lab (Lavender) Spray in Improving Sleep Quality in Insomnia Patients. J Func Vent Pulm 2025;50(16):19-24