
 English
English
 French
French
Laboratory techniques for diagnosis of obstructive sleep apnea (OSA) in children
Techniques laboratoires dans le diagnostique des apnées obstructives du sommeil (AOS) chez les enfants
Y. Nguyen-Hoang1, B. Nguyen-Thi2
1 : Phu Tho General Hospital. Phu Tho province. Viet Nam
2 : Ha Noi University of Medicine. Ha Noi. Viet Nam
Corresponding author
Dr. NGUYEN-HOANG Yen
Phu Tho General Hospital. Phu Tho province. Viet Nam
E-mail: drhoangyenpt@gmail.com
ABSTRACT
Sleep apnea in children has been widely recognized in the past few decades, which is a significant cause of childhood illness and accounts for 1% to 5%. Obstructive sleep apnea (OSA) can lead to serious complications if not detected early and treated promptly. Many studies indicated that children with OSA are at risk of physical retardation, cognitive impairment, such as poor learning, behavioral problems, hyperactivity. Therefore, early detection and diagnosis of OSA is essential to significantly reduce the incidence of childhood illness.
OSA diagnostics in children is continuing to develop and diagnostic methods are becoming more advanced. There are a number of scientifically validated diagnostic methods including: history of the disease, clinical examination, sleep or video recording, oxygen oscillations, X-rays, respiratory syncytial signs and multiple sleep recording. Each diagnostic approach has its own advantages and disadvantages, in which sleep apnea diagnosed by polysomnography (PSG) is considered to be the primary diagnostic criteria for OSA.
Polysomnography rests a main method for assessing the presence and severity of OSA in children. It is important to evaluate all the sleep parameters such as apnea-hypopnea index, abnormal airflow, and the number of respiratory events.
KEYWORDS: Obstructive sleep apnea, apnea-hypopnea index, microarousal.
RÉSUMÉ
L'apnée du sommeil chez les enfants a été largement reconnue au cours des dernières décennies, ce qui est une cause importante de maladie chez les enfants et représente 1% à 5%. Les apnées obstructives du sommeil (AOS) peut entraîner des complications graves si elle n'est pas détectée précocement et traitée rapidement. De nombreuses études ont indiqué que les enfants atteints d'AOS ont eu d’un risque de retard physique, de troubles cognitifs, tels qu'un faible apprentissage, des problèmes de comportement, et une hyperactivité. Par conséquent, la détection précoce et le diagnostic de l'AOS sont essentiels pour réduire significativement l'incidence des maladies infantiles.
Le diagnostic d'AOS chez les enfants continuent à se développer et les méthodes de diagnostic deviennent plus avancées. Il existe un certain nombre de méthodes de diagnostic validées scientifiquement: historique de la maladie, examen clinique, enregistrement du sommeil ou de la vidéo, oscillations de l'oxygène, radiographies, signes syncytiaux respiratoires et recorde de sommeil multiple. Chaque approche diagnostique a ses propres avantages et inconvénients, dans lesquels l'apnée du sommeil diagnostiqué par polysomnographie (PSG) est considérée comme le principal critère diagnostique de l'AOS.
La polysomnographie est la principale méthode d'évaluation de la présence et de la gravité de l'AOS chez l'enfant. Il est important d'évaluer tous les paramètres du sommeil tels que l'indice d'apnée-hypopnée, le flux d'air anormal et le nombre d'événements respiratoires.
MOTS CLÉS: Apnées obstructives du sommeil, index d’apnées-hypopnées, micróeveil.
INTRODUCTION
Obstructive sleep apnea (OSA) is a disorder of respiration during sleep characterized by repeated or partial obstruction of the upper respiratory tract during sleep. resulting in complete respiratory arrest despite respiratory effort [1].
Sleep apnea in children has been widely recognized in the past few decades, which is a significant cause of childhood illness and accounts for 1% to 5% [2]. OSA can lead to serious complications if not detected early and treated promptly. Many studies indicated that children with OSA are at risk of physical retardation, cognitive impairment, such as poor learning, behavioral problems, hyperactivity [1]. Therefore, early detection and diagnosis of OSA is essential to significantly reduce the incidence of childhood illness.
OSA diagnostics in children is continuing to develop and diagnostic methods are becoming more advanced. There are a number of scientifically validated diagnostic methods including: history of the disease, clinical examination, sleep or video recording, oxygen oscillations, x-rays, respiratory syncytial signs and multiple sleep. Each diagnostic approach has its own advantages and disadvantages, in which sleep apnea (polysomnography: PSG) is considered to be the primary diagnostic criteria for OSA.
LABORATORY TECHNIQUES
Endoscopic - endoscopic of larynx
This technique evaluates the structure and function of the upper airway, which directly observes obstructed areas and is usually performed under general anesthesia or anesthesia. Isono et al. used endoscopy to determine the area of maximal airway obstruction in children with OSA [3].
The area is defined as adenoid vegetation (AV) and soft palate. In clinical practice, upper respiratory endoscopy is usually reserved for children with complicated tracheostomy and laryngeal valve closure.
The nasal cavity as well as the oral cavity of children with facial defects previously treated with these defects should be carefully evaluated in a similar manner, as these children may be at risk of recurrence OSA.
Acoustic pharyngometry
Acoustic pharyngometry is a non-invasive method that uses sound waves to measure the area of the upper respiratory tract that has been successfully used in adults but is used in children for very restricted condition [4,5].
X-ray
For evaluation of upper respiratory tract in children with OSA, a number of X-ray techniques were used, including tilted x-rays, skull scans, fluorescents, computerized tomography magnetic resonance imaging (MRI) [6-8]. These methods have proven that the upper airways of children with OSA are smaller than normal children. MRI is particularly useful as it can be used to reconstruct three whole dimensions of the upper airways (including soft tissue and bone structure), as well as assessing coronary motility bronchial asthma [9], but the cost of MRI is high.
The benefits of these techniques differ, some studies have evaluated the benefits of X-rays beyond clinical factors in determining OSA diagnosis. Back radiography is very simple and can be done at the clinic to evaluate the size of the AV and upper airway. The presence of respiratory tract narrowing on the film increased the predictive ability of OSA on PSG. Miniature imaging in patients with OSA compared with controls but also MRI did not confirm this. Nasal resistance was measured using rhinometry, a high sensitivity and specificity to predict OSA on PSG.
Assessment of the function of opening the airways
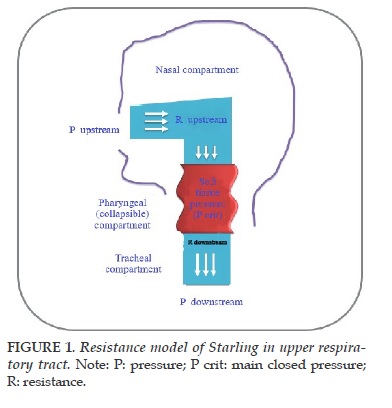
This technique studies the opening of airway in OSA children [10,11]. This model of evaluation is based on a Starling methodology for the idea that considers the upper airways as a open tubbe (Figure 1). Equalation to change of the current flow level on the external application is applied the positive-negative, can only determine the reduce pressure on the airways. This measurement is invoked at the most major of the accuracy of the main closed pressure (Pcrit) and the effect is made by the solution and not resolve. Adults with OSA usually have positive Pcrit, which indicates that the airway will collapse while sleeping due to increase airway pressure even if it is slightly negative [12] if it is not "protected" under impact of this negative pressure from muscle, tissue to expand to the throat. Similar results were found in children, in which Pcrit correlates with the severity of respiratory disorders [13,14]. In addition to the Pcrit measurement, X-ray measurements may be used to evaluate upper airway closure and include also MRI [9,15]. However, nowadays, these techniques are primarily for clinical research and are often not used as a diagnostic tool for OSA in children.
Circuit timing
Electrocardiography has been used to screen OSA in children based on changes in heart rate with respiratory events and ECG-related changes. This technique has the potential as an easy screening tool, but has not been proven in larger OSA studies.
Recording or video
Overnight recording can be used to record snoring, but it is not possible to distinguish between snoring or OSA snoring alone [16]. Therefore, children with positive results should undergo a more comprehensive evaluation to determine the severity and severity of OSA, the study also found that when a child has a negative result may not need to further treatment [17].
Measuring drainage oxygen daily
Detecting decreased oxygen saturation during sleep in children may be due to OSA. However, when considering the difficulty of applying this technique at home to children and in fact many children with increased respiratory resistance have sleepy snoring and wake up without hypoxemia, This technique has significant limitations. Brouillette's study indicates that nighttime oxygen saturation measurements may provide an accurate diagnosis for OSA if positive but if the oxygen result is negative then full PSG is needed [18].
The oxygen score correlates with the AHI index obtained from PSG as well as the presence of respiratory events. However, the positive predictive value of oxygen measurement for respiratory distress after surgery was only 13%. Of note, 80% of the 223 children had normal results, had no results or did not meet the technical requirements and were therefore required to measure oxygen saturation or PSG [18]. In contrast, Kirk compared oxygen measurements at home overnight (using a system with an automatic oxygen analysis algorithm that provided an oxygen saturation index) with PSG measurements in 58 children aged 4 years suspected OSA has been found there was a poor correlation between the oxygen saturation index on the oxygen measurement and AHI as determined by PSG.
Polysomnography (PSG)
Accurate OSA diagnosis based on medical history and physical examination is very low, even if the interview was conducted by a sleep specialist. This relatively low predictability has prompted the recognition and recommendation of children with suspected symptoms assessed for PSG to diagnose or exclude OSA and also to assess the severity of the disease. The American Academy of Pediatrics (AAP) has released a consensus statement stating the requirements for PSG evaluation in children and the recognition of PSG as a diagnostic criteria for establishing diagnostic and therapeutic options. Severity of OSA. Guidelines for the implementation of PSG in children's laboratories have been provided by the American Thoracic Society (ATS). These measures include electroencephalography (EEG) during sleep, and respiratory score records of respiratory events. The American Academy of Sleep Medicine (AASM) has established working groups to study sleep time and evidence of respiratory symptoms and respiratory irritation [19,20] and provide sleep guidelines and record events based on these observations [20].
Different sleep recording types
Recording systems are classified based on the number of sensors and recording conditions:
-Type I: Supervised sleep laboratory sleep with trained staff and at least 7 signals: Electroencephalogram (EEG), Electroocculogram (EOG), Electromyogram (EMG), nasal-air flow, respiratory effort, ECG, oxygen saturation, posture, snoring.
-Type II: Multi-sleep sleep in unattended condition: with at least 7 signals and measured at home.
-Type III: Respiratory device with at least 4 signals: Nasal airflow volume one or two signals of respiratory activity, oxygen saturation and cardiac or ECG for cardiac frequency.
Type IV: One or two most common respiratory signs are oxygen saturation and/or gas flow (Figure 2).
Sleep enrollees performed in sleep centers (Type I) are the primary test for the diagnosis of apnea syndrome - sleep apnea as it provides an objective quantitative assessment of respiratory disorders steam and sleep. It has been demonstrated that there are the relationships between PSG and sequelae of the OSA finding that PSG allows patients to be stratified by severity, identifying those at risk for diurnal; therefore, warning pediatricians to check OSA complications and those at risk for postoperative complications should be monitored for outpatient follow-up. After surgery, PSG should be screened after surgery to assess the need for further treatment.
Adults may fall asleep the first time they sleep in a sleep study room because of anxiety, unfamiliar environment, and sensor attached. This "first night effect" can lead to a variable sleep structure and may underestimate the severity of the OSA. Many previous studies also evaluated nighttime PSG changes in children showed that there was only a small variation was evaluated at night.
However, this technique is expensive, time consuming and not available in many health facilities. Also note that sleep is not all that determines treatment in OSA.
Sleep-dependent monitoring (Type II) is more convenient than Type I because it can be done during daytime (noon), at home, more convenient for patients and no need for visiting sleep staff. Studies have shown a positive predictive value of 77% to 100% and a negative predictive value of 17% to 49% [17,22]. In children with OSA, PSG overnight (Type I) showed more severe abnormalities than Type II studies.
Therefore, unmonitored PSG may be useful if the results are positive although it may underestimate the severity of the OSA. An overnight PSG study should be performed if PSG is not monitored for negative results. The difference may be due to decreased REM sleep during nap, as well as reduced sleep time. Uncontrollable sleep over night at home has not been studied much. Unrecognized rates of failure due to technical failures in these conditions and up to 20% [23].
The diagnostic efficacy of Type III was comparable to that of Type I sleep. These studies gave the former results of obstructive sleep apnea syndrome in patients diagnosed clinically. If obstructive sleep apnea syndrome is suspected, clinical suspicion may be required to perform multiple sleep recordings.
False negative results of obstructive sleep apnea may be explained by inadequate evaluation of the apnea-reduction index because of lack of sleep time and/or failure to recognize events without sedation.
The changeable nature of the night may also explain some of the false negative results, especially in moderate OSA and need to be reassessed with a second recording in case of suspected and clinically significant [23].
Respiratory evaluation is offered as a first-line clinical diagnosis of OSA and no data is available to suggest other sleep disorders.
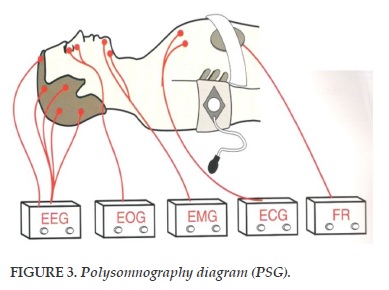
There are many variables that are tracked and recorded in the PSG, but there is no need to apply all to the diagnosis (Figure 3).
Electroencephalogram (EEG): An analysis of sleep patterns that suggests that a sleep has sufficient total sleep time and that adequate REM sleep is obtained from the night of study to demonstrate the presence or absence of stool. piece of sleep. In addition, EEG also records cortical excitations and seizures. One study found that sleep disorders are present in 51% of childhood congestive events. A large cohort study (n=559) found that children with OSA had a slower and shorter fall in REM sleep than did normal children.
Electrooculoglogram (EOG): The movement of the eye from the outer electrodes of each eye: rapid eye movement detection.
Electromyogram (EMG): From electrodes placed on the posture: chin and leg muscles.
Electrocardiogram (ECG) with one or more electrodes in the chest.
Respiratory tightness, through the examination of chest and abdominal wall motion with a belt fitted with a sensor.
Airflow through the nose and/or mouth through the thermometer and nasal probe.
Oxygen saturation (SpO2) through a waveform pulse with an average duration of no more than 3 seconds.
CO2 measurement (PaCO2) or CO2 through the skin (Ptc CO2).
Location of the body through the sensor and direct observation.
Foot movement (right and left leg) via EMG.
Snoring or vibration (frequency and/or volume). Video/audio recording with infrared or low light.
Respiratory events
Obstructive apnea
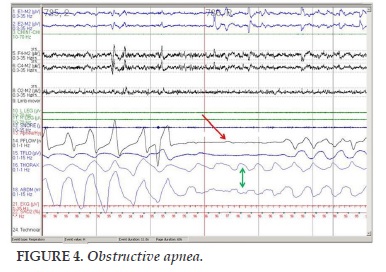
An obstructive apnea is recognized to have decreased > 90% or complete loss of signal amplitude of airflow for respiratory events than amplitude baseline before, and this event lasts for at least two breaths or the duration of two basic breaths, (stopping air-mouth flow for at least 10 seconds) with effort breathing at the end of the apnea. Interval of apnea is calculated from the end of the last breath to the beginning of the first breath (Figure 4).
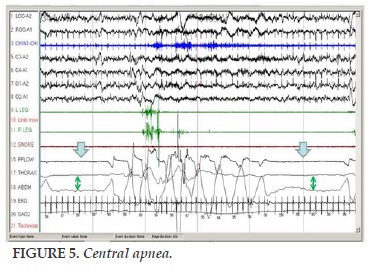
Central apnea
It is defined as stop nasal air flow at least 10 seconds and no respiratory effort when breathing air (Figure 5).
Mixture apnea
An event respiratory marked as a mixture apnea if signal airflow lost a breathing time and standard amplitude of respiratory as the same with obstructive apnea; but an mixture apnea manifests initially such as central respiratory arrest but ended with respiratory exertion.
Hypopnea
There is no clear definition of breathing reduction. This event can be recorded with a reduction of the air flow velocity for at least 10 seconds and with one of the following criteria:
Reduced airflow signal amplitude >50% initial margin to at least 90% of the time of the event.
Decrease of less than 50% or a plateau curve is accompanied by a reduction in oxygen saturation through the skin by at least 3% and/or wakefulness.
Micro arousal
A potential signal, as only the best of pathology occurs during sleep: It is a general sensitive event for apnea, decreased breathing and periods with increased respiratory effort. This is an important factor for diagnosing and reducing the number of times waking up is a treatment goal.
Awakening is a sudden change in EEG frequencies, abnormal waves θ, α occur in slow sleep, fast β waves in the dormant period, and/or frequency greater than 16 Hz, lasting at least 3 seconds, with at least 10 seconds of sleep stable before the change. Recording of excitement during the REM process requires an increase in the accompanying brain waves for at least 1 second. Sleep disturbances can be spontaneous or may involve events such as a technician's intervention, respiratory events and limb movements.
Micro arousal related to respiratory effort
An awakening related to respiration can be recognized when an event associated with the snoring, breathing heavy, increasing PET CO2 /Ptc CO2 , or visual evidence that increases breathing, and that the event lasts for at least two breath cycles (or initial breathing time of two breaths) if one of the following occurs: 1) The pressure in the nasal air pressure sensor is 50% lower than the baseline and the nasal pressure plateau curve; or 2) there is an increase in expiratory breath during the event with decreased oesophageal pressure ascending.
Hypoventilation
It is recognized when over 25% of total sleep time with CO2 >50 mmHg, as measured by the sensor through the skin Ptc CO2 and/or PET CO2.
Index of apnea
Total obstructive and/or centeral apnea of each hour of sleep.
Index of obstructive apnea
Number of times per hour obstructive sleep apnea.
Index of decreased breathing (hypopnea index - HI)
Number of times per hour of sleep reduced breathing.
Index of apnea - hypopnea (apnea-hypopnea index - AHI ) General index of apnea and hypopnea per hour of sleep.
Obstructive apnea-hypopnea index (OAHI)
Total obstructive sleep apnea and respiratory hypopnea.
Syndrome increased upper airway resistance
A respiratory disorders related to snoring sleep, causing excessive daytime sleepiness, fragmentation wake up and sleep.
Electroencephalography and microwaves in sleep
Respiratory events during sleep can lead to an increase in the number and duration of waking that can cause sleep disturbances and disrupt normal sleep structure, resulting in neurological consequences and behavior, such as increased sleepiness and decreased attention. However, in children, this is not always proven. Children from mild to moderate OSA may have no change in wakefulness index, but increased levels of sleep apnea with increasing excitement in children .The sleep pattern may shows slower sleep and REM sleep than the control group, but is still normal, unless there is severe sleep apnea. In a recent study, sleep deprivation scores were found to show that sleep deprivation scores measured by microarousal showed sleepiness relative to severity of OSA in children. And the sleep deprivation score assessed by the awake index was negatively correlated with the neuropsychological capacity in OSA infants.
In addition to analyzing changes in EEG relative to respiratory events in sleep, changes in hemodynamic functions, such as changes in heart rate and blood pressure, have also been reported. In fact, these symptoms are more common than brain electrical excitation and may be more sensitive measures for detecting cortical stimuli and predicting neurological and/or cardiovascular consequences in children with OSAS.
Other measurement techniques
ATS published 1996 consensus guidelines for the recording of respiratory phenomena, including apnea (without nasobucal airflow) and respiratory depression (reduced nasal airflow), for congestive and central events, reduced ventilation and reduced oxygen saturation. These guidelines have been replaced by recent recommendations ("Rules") for the measurement and recording of respiratory events in children (infants up to 18 years of age) under the AASM [25]. The detection and clarification of abnormal respiratory events required the collection and recording of significant signs of respiratory flows and events.
Time of pulse transit
The pulse transit time (PTT) estimates the time interval between the pulses causing the pressure of the vessel at the root of the aorta until it appears at the periphery. This is measured by the time between the R waves on the ECG and the detection of finger circuits by spectral measurements. PTT is inversely proportional to blood pressure. High blood pressure, combined with respiratory stimulation from sleep, leads to a decrease in PTT. Katz et al. reported that the number of boosting stimuli of PTT (6.8/h) was higher than the EEG stimulus (2.2/h) in children with increased respiratory resistance [ 24]. The importance of this approach in children with sleep disturbance (SBD) and OSA to predict unpublished clinical outcomes.
Peripheral arterial pressure measurement.
Peripheral arterial tonometry (PAT) measurements of finger pulse are connected to a blood pressure cuff enclosing the fingers with a uniform pressure.
Increased vasoconstriction, resulting in reduced pulse amplitude. PAT provides a measure of automatic changes with sleep microphones. Similar to PTT, PAT may be more sensitive to infant than changes in EEG.
Respiratory and esophageal pressure. The measurement of nose-mouth by pneumotachography and continuous measurement of esophageal pressure are two optimal methods but rarely applied in practice.
Nose-mouth thermistor. Best tool to detect apnea but have insufficient sensitivity for detecting hypopnea.
Nasal pressure gauge. The detection of nasal obstruction has been demonstrated in comparison with the standard method. In the case of weak nasal pressure, total chest and abdominal movements assessed by systemic spirometry will contribute to the detection of hypopnea.
Heat resistance. A pressure sensor switch nose. For apnea and respiratory arrest, use of nasal pressure measurement combined with a sensor to detect whether or not breathing by mouth: thermal resistance to the mouth or trachea. Do not use solitary heat sensor to detect respiratory events.
Waste capacitance touch up. The assessment of respiratory effort is based on motion detection chest-abdomen through spirometry touch up. The complete absence of complete movement of the chest and abdomen when apnea is indicated by central origin. The obstruction characteristic of hypothermia is manifested by the phase shift of the abdominal-abdominal movement. The amplitude response of the electronic sensor is not linear.
Pressure on the sternum. Measurement (non-invasive) pressure on the sternum helps to classify the type of apnea.
Measurement of oxygen saturation. The reduction of hemoglobin saturation with oxygen included in the determination of some kind of breath decrease. Thus, the ability to use oxygen saturation instruments in detecting reduced oxygen saturation in a few seconds affects the calculation of the respiratory rate reduction.
The average window time, sample frequency, and response time are the major determinants of the performance of oxygen saturation measurements and their effect on performance. Use an oxygen saturation meter with a high sample frequency suitable for an average window with a maximum period of 3-5 seconds.
Biomarkers of OSA
Biomarkers are studied from the evaluation of blood, urine, saliva, and possibly in exhaled air, which were thought to be related to OSA. Graziela De Luca Canto and colleagues report that IL-6 and IL-10 have the potential to be a good biomarker for determining whether or not to have OSA in adults. While the combination of kallikrrein-1, uromodulin, urocortin-3 and orosomucoid-1 seems to be accurate enough to be considered as a diagnostic test for OSA in children [26]. Increased CRP levels have been demonstrated in children with OSA, correlated with the severity of the disease and decreased after effective treatment [27]. It should be emphasized that not all children with OSA have high CRP levels because of the interaction of genetic variants in the IL-6 and CRP genes as well as environmental factors that play an important role [27].
Increased CRP levels were also associated with an increased risk of cognitive decline in OSA children.
In a Vietnamese study, Duong-Quy et al. reported that OSA alveolar nitric oxide (CANO) concentrations significantly increased in OSA compared to before sleep [28]. The author suggests that measuring CANO at waking-up can be considered as a biomarker of subjects with OSA. However, no conclusions have yet been made regarding the validity of the biomarker test in diagnosis of OSA. Therefore, biomarkers of OSA needs more studies in the future.
CONCLUSION
Polysomnography rests a main method for assessing the presence and severity of OSA in children. It is important to evaluate all the sleep parameters such as apnea-hypopnea index, abnormal air exchange, and the number of respiratory events. Therefore, when any of these indicator parameters are abnormal, the diagnosis of OSA and its clinical treatment should be based on sleep structure, sleep history, and outcome of clinical symptoms [29-34]. The choice of suitable techniques in study of OSA and their sleep pattern is still necessary.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
1. Isono, S., et al., Comparison of static mechanical properties of the passive pharynx between normal children and chil-dren with sleep-disordered breathing. Am J Respir Crit Care Med, 1998. 157(4 Pt 1): p. 1204-12.
2. Lumeng, J.C. and R.D. Chervin, Epidemiology of pediat-ric obstructive sleep apnea. Proc Am Thorac Soc, 2008. 5(2): p. 242-52.
3. Rosen, G.M., et al., Postoperative respiratory compromise in children with obstructive sleep apnea syndrome: can it be anticipated? Pediatrics, 1994. 93(5): p. 784-8.
4. Fregosi, R.F., et al., Sleep-disordered breathing, pharynge-al size and soft tissue anatomy in children. J Appl Physiol 2003. 95(5): p. 2030-8.
5. O'Brien, L.M., R. Tauman, and D. Gozal, Sleep pressure correlates of cognitive and behavioral morbidity in snoring children. Sleep, 2004. 27(2): p. 279-82.
6. Franklin, K.A. and E. Lindberg, Obstructive sleep apnea is a common disorder in the population-a review on the epidemiology of sleep apnea. J Thorac Dis, 2015. 7(8): p. 1311-22.
7. Monahan, K.J., et al., Utility of noninvasive pharyngome-try in epidemiologic studies of childhood sleep-disordered breathing. Am J Respir Crit Care Med, 2002. 165(11): 1499-503.
8. Stradling, J.R., et al., Effect of adenotonsillectomy on noc-turnal hypoxaemia, sleep disturbance, and symptoms in snoring children. Lancet, 1990. 335(8684): p. 249-53.
9. Marcus, C.L., et al., Diagnosis and management of child-hood obstructive sleep apnea syndrome. Pediatrics, 2012. 130(3): p. e714-55.
10. Marcus, C.L., et al., Developmental changes in response to subatmospheric pressure loading of the upper airway. J Appl Physiol (1985), 1999. 87(2): p. 626-33.
11. Gleadhill, I.C., et al., Upper airway collapsibility in snorers and in patients with obstructive hypopnea and apnea. Am Rev Respir Dis, 1991. 143(6): p. 1300-3.
12. Gozal, D. and M.M. Burnside, Increased upper airway collapsibility in children with obstructive sleep apnea during wakefulness. Am J Respir Crit Care Med, 2004. 169(2): p. 163-7.
13. Grigg-Damberger, M.M., The AASM Scoring Manual four years later. J Clin Sleep Med, 2012. 8(3): p. 323-32.
14. Scholle, S. and G. Zwacka, Arousals and obstructive sleep apnea syndrome in children. Clin Neurophysiol, 2001. 112(6): p. 984-91.
15. Goldstein, N.A., et al., Clinical diagnosis of pediatric ob-structive sleep apnea validated by polysomnography. Oto-laryngol Head Neck Surg, 1994. 111(5): p. 611-7.
16. Grigg-Damberger, M., et al., The visual scoring of sleep and arousal in infants and children. J Clin Sleep Med, 2007. 3(2): p. 201-40.
17. Kawashima, S., et al., Cephalometric comparisons of crani-ofacial and upper airway structures in young children with obstructive sleep apnea syndrome. Ear Nose Throat J, 2000. 79(7): p. 499-502, 505-6.
18. Hunter, S.J. and D. Gozal, Effect of Sleep-disordered Breathing Severity on Cognitive Performance Measures in a Large Community Cohort of Young School-aged Children. 2016. 194(6): p. 739-47.
19. Muzumdar, H. and R. Arens, Diagnostic issues in pediat-ric obstructive sleep apnea. Proc Am Thorac Soc, 2008. 5(2): p. 263-73.
20. Arens, R., et al., Changes in upper airway size during tidal breathing in children with obstructive sleep apnea syndrome. Am J Respir Crit Care Med, 2005. 171(11): p. 1298-304.
21. Duong-Quy S, Dang Thi Mai K, Tran Van N, et al. Study about the prevalence of the obstructive sleep apnoea syndrome in Vietnam. Rev Mal Respir. 2018 Jan;35(1):14-24.
22. Arens, R., et al., Magnetic resonance imaging of the upper airway structure of children with obstructive sleep apnea syndrome. Am J Respir Crit Care Med, 2001. 164(4): p. 698-703.
23. Marcus, C.L., et al., Developmental changes in upper air-way dynamics. J Appl Physiol (1985), 2004. 97(1): p. 98-108.
24. Shouldice, R.B., et al., Detection of obstructive sleep apnea in pediatric subjects using surface lead electrocardiogram features. Sleep, 2004. 27(4): p. 784-92.
25. Huang, J., et al., Upper airway collapsibility during REM sleep in children with the obstructive sleep apnea syndrome. Sleep, 2009. 32(9): p. 1173-81.
26. Bonnet, M.H., et al., The scoring of arousal in sleep: relia-bility, validity, and alternatives. J Clin Sleep Med, 2007. 3(2): p. 133-45.
27. O'Brien, L.M., R. Tauman, and D. Gozal, Sleep pressure correlates of cognitive and behavioral morbidity in snoring children. Sleep, 2004. 27(2): p. 279-82.
28. S Duong-Quy, Thong Hua-Huy, Huyen-Tran Tran-Mai-Thi, Nhat-Nam Le-Dong, Timothy J. Craig, Anh-Tuan Dinh-Xuan. Study of Exhaled Nitric Oxide in Subjects with suspected Obstructive Sleep Apnea: A Pilot Study in Vietnam. Pulmonary Medicine 2016, Article ID 3050918, 7 pages.
29. Duong-Quy S. Sleep disorder in COPD: a forgotten entity. J Func Vent Pulm 2015;19(6):1.
30. Nguyen-Thi-Hong L, Duong-Quy S. Obstructive Sleep Apnea Syndrome: The challenges in developing coun-tries. J Func Vent Pulm 2016;22(7):1-2.
31. Dang Thi Mai K, Tran Van N. Study of the prevalence of metabolic syndrome in patients with sleep apnea syndrome. J Func Vent Pulm 2013;04(10):36-42.
32. Nguyen Xuan Bich H. Obstructive sleep apnea syn-drome (OSAS) and arterial hypertension. J Func Vent Pulm2014;05(14):1-2.
33. Hua-Huy T. Obstructive sleep apnoea syndrome (OSAS): on the right method for screening . J Func Vent Pulm 2014;05(15):3-4.
34. Martin F, Duong-Quy S. Sleep apnea syndrome in dai-ly practice: Welcome to the 2nd edition of sleep disor-der book in French - Vietnamese languages. J Func Vent Pulm 2016; 21(7): 1-2.
FIGURES
REFERENCES
1. Isono, S., et al., Comparison of static mechanical properties of the passive pharynx between normal children and chil-dren with sleep-disordered breathing. Am J Respir Crit Care Med, 1998. 157(4 Pt 1): p. 1204-12.
2. Lumeng, J.C. and R.D. Chervin, Epidemiology of pediat-ric obstructive sleep apnea. Proc Am Thorac Soc, 2008. 5(2): p. 242-52.
3. Rosen, G.M., et al., Postoperative respiratory compromise in children with obstructive sleep apnea syndrome: can it be anticipated? Pediatrics, 1994. 93(5): p. 784-8.
4. Fregosi, R.F., et al., Sleep-disordered breathing, pharynge-al size and soft tissue anatomy in children. J Appl Physiol 2003. 95(5): p. 2030-8.
5. O'Brien, L.M., R. Tauman, and D. Gozal, Sleep pressure correlates of cognitive and behavioral morbidity in snoring children. Sleep, 2004. 27(2): p. 279-82.
6. Franklin, K.A. and E. Lindberg, Obstructive sleep apnea is a common disorder in the population-a review on the epidemiology of sleep apnea. J Thorac Dis, 2015. 7(8): p. 1311-22.
7. Monahan, K.J., et al., Utility of noninvasive pharyngome-try in epidemiologic studies of childhood sleep-disordered breathing. Am J Respir Crit Care Med, 2002. 165(11): 1499-503.
8. Stradling, J.R., et al., Effect of adenotonsillectomy on noc-turnal hypoxaemia, sleep disturbance, and symptoms in snoring children. Lancet, 1990. 335(8684): p. 249-53.
9. Marcus, C.L., et al., Diagnosis and management of child-hood obstructive sleep apnea syndrome. Pediatrics, 2012. 130(3): p. e714-55.
10. Marcus, C.L., et al., Developmental changes in response to subatmospheric pressure loading of the upper airway. J Appl Physiol (1985), 1999. 87(2): p. 626-33.
11. Gleadhill, I.C., et al., Upper airway collapsibility in snorers and in patients with obstructive hypopnea and apnea. Am Rev Respir Dis, 1991. 143(6): p. 1300-3.
12. Gozal, D. and M.M. Burnside, Increased upper airway collapsibility in children with obstructive sleep apnea during wakefulness. Am J Respir Crit Care Med, 2004. 169(2): p. 163-7.
13. Grigg-Damberger, M.M., The AASM Scoring Manual four years later. J Clin Sleep Med, 2012. 8(3): p. 323-32.
14. Scholle, S. and G. Zwacka, Arousals and obstructive sleep apnea syndrome in children. Clin Neurophysiol, 2001. 112(6): p. 984-91.
15. Goldstein, N.A., et al., Clinical diagnosis of pediatric ob-structive sleep apnea validated by polysomnography. Oto-laryngol Head Neck Surg, 1994. 111(5): p. 611-7.
16. Grigg-Damberger, M., et al., The visual scoring of sleep and arousal in infants and children. J Clin Sleep Med, 2007. 3(2): p. 201-40.
17.Kawashima, S., et al., Cephalometric comparisons of crani-ofacial and upper airway structures in young children with obstructive sleep apnea syndrome. Ear Nose Throat J, 2000. 79(7): p. 499-502, 505-6.
18. Hunter, S.J. and D. Gozal, Effect of Sleep-disordered Breathing Severity on Cognitive Performance Measures in a Large Community Cohort of Young School-aged Children. 2016. 194(6): p. 739-47.
19. Muzumdar, H. and R. Arens, Diagnostic issues in pediat-ric obstructive sleep apnea. Proc Am Thorac Soc, 2008. 5(2): p. 263-73.
20. Arens, R., et al., Changes in upper airway size during tidal breathing in children with obstructive sleep apnea syndrome. Am J Respir Crit Care Med, 2005. 171(11): p. 1298-304.
21. Duong-Quy S, Dang Thi Mai K, Tran Van N, et al. Study about the prevalence of the obstructive sleep apnoea syndrome in Vietnam. Rev Mal Respir. 2018 Jan;35(1):14-24.
22. Arens, R., et al., Magnetic resonance imaging of the upper airway structure of children with obstructive sleep apnea syndrome. Am J Respir Crit Care Med, 2001. 164(4): p. 698-703.
23. Marcus, C.L., et al., Developmental changes in upper air-way dynamics. J Appl Physiol (1985), 2004. 97(1): p. 98-108.
24. Shouldice, R.B., et al., Detection of obstructive sleep apnea in pediatric subjects using surface lead electrocardiogram features. Sleep, 2004. 27(4): p. 784-92.
25. Huang, J., et al., Upper airway collapsibility during REM sleep in children with the obstructive sleep apnea syndrome. Sleep, 2009. 32(9): p. 1173-81.
26. Bonnet, M.H., et al., The scoring of arousal in sleep: relia-bility, validity, and alternatives. J Clin Sleep Med, 2007. 3(2): p. 133-45.
27. O'Brien, L.M., R. Tauman, and D. Gozal, Sleep pressure correlates of cognitive and behavioral morbidity in snoring children. Sleep, 2004. 27(2): p. 279-82.
28. S Duong-Quy, Thong Hua-Huy, Huyen-Tran Tran-Mai-Thi, Nhat-Nam Le-Dong, Timothy J. Craig, Anh-Tuan Dinh-Xuan. Study of Exhaled Nitric Oxide in Subjects with suspected Obstructive Sleep Apnea: A Pilot Study in Vietnam. Pulmonary Medicine 2016, Article ID 3050918, 7 pages.
29. Duong-Quy S. Sleep disorder in COPD: a forgotten entity. J Func Vent Pulm 2015;19(6):1.
30. Nguyen-Thi-Hong L, Duong-Quy S. Obstructive Sleep Apnea Syndrome: The challenges in developing coun-tries. J Func Vent Pulm 2016;22(7):1-2.
31. Dang Thi Mai K, Tran Van N. Study of the prevalence of metabolic syndrome in patients with sleep apnea syndrome. J Func Vent Pulm 2013;04(10):36-42.
32. Nguyen Xuan Bich H. Obstructive sleep apnea syn-drome (OSAS) and arterial hypertension. J Func Vent Pulm2014;05(14):1-2.
33. Hua-Huy T. Obstructive sleep apnoea syndrome (OSAS): on the right method for screening . J Func Vent Pulm 2014;05(15):3-4.
34. Martin F, Duong-Quy S. Sleep apnea syndrome in dai-ly practice: Welcome to the 2nd edition of sleep disor-der book in French - Vietnamese languages. J Func Vent Pulm 2016; 21(7): 1-2.
ARTICLE INFO
DOI: 10.12699/jfvpulm.9.27.2018.3
Conflict of Interest
Non
Date of manuscript receiving
12/03/2018
Date of publication after correction
30/08/2018
Article citation
Nguyen-Hoang Y, Nguyen-Thi B. Laboratory techniques for diagnosis of obstructive sleep apnea (OSA) in children. J Func Vent Pulm 2018;27(9):3-10